Abstract
Aim
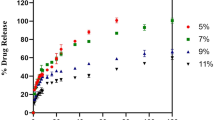
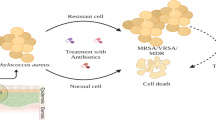
Meropenem hydrochloride (MpM)-loaded nanostructured lipid carriers were designed for the effective management of skin infection caused by Staphylococcus aureus via topical route. The solvent evaporation tactic was preferred to develop nanostructured lipid carriers (NLCs). Stearic acid was used as a solid fatty acid; oleic acid was used as liquid fatty acid and Tween 80 as a surfactant. The Staphylococcus aureus burden was analyzed by pharmacodynamic studies. The skin retention was analyzed by fluorescence microscopy. Spherical shape of NLCs was confirmed by TEM. The optimum particle size of the MpM-NLCs was ~ 126.5 ± 0.9 nm with 79.1 ± 2.3% entrapment (EE) and 0.967 mV zeta potential. The in vitro release studies revealed 81.5 ± 3.1% release of drug in 48 h, while the pure drug was almost completely released (98.4 ± 1.4%) within 24 h confirming the potential of NLCs for sustained topical drug delivery. Skin permeation study also revealed better permeation of drug from NLCs than of plain drug. The prepared MpM-NLCs when stored at 4 ± 2°C for 90 days were found to be more stable when the formulation was stored at 28 ± 2°C. The S. aureus burden was analyzed by evaluating the zone of inhibition (ZOI). The ZOI of MpM alone and MpM-NLC gel was measured and compared with that of the control group. The MpM was found significantly effective when its gel was prepared with NLCs because of its enhanced adhesion property occlusion and ability to sustain release. In overall, the study’s outcomes validated the relevance of NLC’s composition as a vehicle for topical MpM administration in skin diseases caused by Staphylococcus aureus.






Similar content being viewed by others
Abbreviations
- BBD:
-
Box-Behnken design
- CLSM:
-
Confocal laser scanning microscopic
- DOE:
-
Design of experiment
- EE:
-
Entrapment efficiency
- FITC:
-
Fluorescein isothiocyanate
- MpM:
-
Meropenem
- NLCs:
-
Nanostructured lipid carriers
- SSTI:
-
Skin and soft tissue infections
- TEM:
-
Transmission electron microscope
- USFDA:
-
US Food and Drug Administration
- WHO:
-
World Health Organization
- ZOI:
-
Zone of inhibition
References
Dryden MS. Skin and soft tissue infection: microbiology and epidemiology. Int J Antimicrob Agents. 2009;34(SUPPL. 1):S2–7. https://doi.org/10.1016/S0924-8579(09)70541-2.
Kaye KS, Petty LA, Shorr AF, Zilberberg MD. Current epidemiology, etiology, and burden of acute skin infections in the United States. Clin Infect Dis. 2019;68(Suppl 3):S193–S9. https://doi.org/10.1093/cid/ciz002.
Barbier F, Timsit JF. Risk stratification for multidrug-resistant bacteria in patients with skin and soft tissue infection. Curr Opin Infect Dis. 2020;33(2):137–45. https://doi.org/10.1097/QCO.0000000000000642.
Chifiriuc MC, Holban AM, Curutiu C, Ditu L-M, Mihaescu G, Oprea AE, et al. Antibiotic drug delivery systems for the intracellular targeting of bacterial pathogens. Smart Drug Delivery System. IntechOpen; 2016.
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–24.
Yeh Y-C, Huang T-H, Yang S-C, Chen C-C, Fang J-Y. Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances. Front Chem. 2020;8:286.
Prajapati SK, Jain A, Shrivastava C, Jain AK. Hyaluronic acid conjugated multi-walled carbon nanotubes for colon cancer targeting. Int J Biol Macromol. 2019;123:691–703.
Prajapati SK, Mishra G, Malaiya A, Kesharwani P, Mody N, Jain A. Application of coatings with smart functions. Mini-Reviews in Organic Chemistry. 2021;18(7):943–60.
Shrestha H, Bala R, Arora S. Lipid-based drug delivery systems. J Pharm 2014;2014.
Chauhan I, Yasir M, Verma M, Singh AP. Nanostructured lipid carriers: a groundbreaking approach for transdermal drug delivery. Advanced Pharmaceutcial Bulletin. 2020;10(2):150–65. https://doi.org/10.34172/apb.2020.021.
Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 2018;13(4):288.
Fernandes AV, Pydi CR, Verma R, Jose J, Kumar L. Design, preparation and in vitro characterizations of fluconazole loaded nanostructured lipid carriers. Braz. J Pharm Sci. 2020;56:18069. https://doi.org/10.1590/s2175-97902019000318069.
Sanad RA, Abdelmalak NS, Elbayoomy TS, Badawi AA. Formulation of a novel oxybenzone-loaded nanostructured lipid carriers (NLCs). AAPS PharmSciTech. 2010;11(4):1684–94. https://doi.org/10.1208/s12249-010-9553-2.
Nobari Azar FA, Pezeshki A, Ghanbarzadeh B, Hamishehkar H, Mohammadi M. Nanostructured lipid carriers: promising delivery systems for encapsulation of food ingredients. J Agric Food Res. 2020;2:100084. https://doi.org/10.1016/j.jafr.2020.100084.
Babazadeh A, Ghanbarzadeh B, Hamishehkar H. Novel nanostructured lipid carriers as a promising food grade delivery system for rutin. J Funct Foods. 2016;26:167–75. https://doi.org/10.1016/j.jff.2016.07.017.
Fish DN. Meropenem in the treatment of complicated skin and soft tissue infections. Ther Clin Risk Manag. 2006;2(4):401–15. https://doi.org/10.2147/tcrm.2006.2.4.401.
Mohr JF III. Update on the efficacy and tolerability of meropenem in the treatment of serious bacterial infections. Clin Infect Dis. 2008;47(Supplement_1):S41–51.
Mhango EKG, Kalhapure RS, Jadhav M, Sonawane SJ, Mocktar C, Vepuri S, et al. Preparation and optimization of meropenem-loaded solid lipid nanoparticles: in vitro evaluation and molecular modeling. AAPS PharmSciTech. 2017;18(6):2011–25. https://doi.org/10.1208/s12249-016-0675-z.
Nava-Arzaluz MG, Piñón-Segundo E, Ganem-Rondero A. Lipid nanocarriers as skin drug delivery systems. In: Grumezescu AM, editor. Nanoparticles in pharmacotherapy. Elsevier; 2019. p. 311-90.
Patel D, Patel B, Thakkar H. Lipid based nanocarriers: promising drug delivery system for topical application. Eur J Lipid Sci Technol. 2021;123:2000264. https://doi.org/10.1002/ejlt.202000264.
Prajapati SK, Mishra G, Malaiya A, Jain A, Mody N, Raichur AM. Antimicrobial application potential of phytoconstituents from turmeric and garlic. In: Pal D, Nayak AK, editors. Bioactive natural products for pharmaceutical applications: Springer Science and Business Media Deutschland GmbH; 2021. p. 409–35.
Hu FQ, Jiang SP, Du YZ, Yuan H, Ye YQ, Zeng S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf B: Biointerfaces. 2005;45(3-4):167–73. https://doi.org/10.1016/j.colsurfb.2005.08.005.
Jain A, Mehra NK, Nahar M, Jain NK. Topical delivery of enoxaparin using nanostructured lipid carrier. J Microencapsul. 2013;30(7):709–15. https://doi.org/10.3109/02652048.2013.778908.
Al-Qushawi A, Rassouli A, Atyabi F, Peighambari SM, Esfandyari-Manesh M, Shams GR, et al. Preparation and characterization of three tilmicosin-loaded lipid nanoparticles: physicochemical properties and in-vitro antibacterial activities. Iran J Pharm Res. 2016;15(4):663–76.
Prajapati SK, Kesharwani P, Mody N, Jain A, Sharma S. Formulation by design (FbD): an emerging approach to design vesicular nanocarriers. In: Mehra NK, Gulbake A, editors. Micro- and nanotechnologies-based product development. 1st ed.: CRC Press; 2021. p. 15-31.
Moghddam SMM, Ahad A, Aqil M, Imam SS, Sultana Y. Optimization of nanostructured lipid carriers for topical delivery of nimesulide using Box–Behnken design approach. Artif Cells Nanomed Biotechnol. 2017;45(3):617–24. https://doi.org/10.3109/21691401.2016.1167699.
Pradhan M, Yadav K, Singh D, Singh MR. Topical delivery of fluocinolone acetonide integrated NLCs and salicylic acid enriched gel: a potential and synergistic approach in the management of psoriasis. J Drug Deliv Sci Technol. 2021;61:102282. https://doi.org/10.1016/j.jddst.2020.102282.
Prajapati V, Jain A, Jain R, Sahu S, Kohli DV. Treatment of cutaneous candidiasis through fluconazole encapsulated cubosomes. Drug Deliv Transl Res. 2014;4(5-6):400–8. https://doi.org/10.1007/s13346-014-0202-2.
Jain A, Jain S, Jain R, Kohli DV. Coated chitosan nanoparticles encapsulating caspase 3 activator for effective treatment of colorectral cancer. Drug Deliv Transl Res. 2015;5(6):596–610. https://doi.org/10.1007/s13346-015-0255-x.
Gaba B, Fazil M, Khan S, Ali A, Baboota S, Ali J. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull Fac Phar Cairo Univ. 2015;53(2):147–59. https://doi.org/10.1016/j.bfopcu.2015.10.001.
Phatak AA, Chaudhari PD. Development and evaluation of nanostructured lipid carrier (NLC) based topical delivery of an anti-inflammatory drug. J Pharm Res. 2013;7(8):677–85.
Cirri M, Bragagni M, Mennini N, Mura P. Development of a new delivery system consisting in “drug–in cyclodextrin–in nanostructured lipid carriers” for ketoprofen topical delivery. Eur J Pharm Biopharm. 2012;80(1):46–53.
Khan S, Jain P, Sourabh J, Richa J, Jain A. Topical delivery of erythromycin through cubosomes for acne. Pharm Nanotechnol. 2018;06(1). https://doi.org/10.2174/2211738506666180209100222
Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1994;82(3):377–90.
Jain S, Prajapati SK, Jain S, Jain S, Jain A. Propylene glycol-liposome for anticoagulant drug delivery through skin. J Bionanosci. 2018;12(5):721–7.
Khan UA, Rahman H, Niaz Z, Qasim M, Khan J, Tayyaba, et al. Antibacterial activity of some medicinal plants against selected human pathogenic bacteria. Eur J Microbiol Immunol. 2013;3(4):272–4. https://doi.org/10.1556/eujmi.3.2013.4.6.
Thirumurugan G, Seshagiri Rao JVLN, Dhanaraju MD. Elucidating pharmacodynamic interaction of silver nanoparticle - topical deliverable antibiotics. Sci Rep. 2016;6(1):1–11. https://doi.org/10.1038/srep29982.
Liu J, Gong T, Wang C, Zhong Z, Zhang Z. Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: preparation and characterization. Int J Pharm. 2007;340(1-2):153–62. https://doi.org/10.1016/j.ijpharm.2007.03.009.
Zhao S, Minh LV, Li N, Garamus VM, Handge UA, Liu J, et al. Doxorubicin hydrochloride-oleic acid conjugate loaded nanostructured lipid carriers for tumor specific drug release. Colloids Surf B: Biointerfaces. 2016;145:95–103. https://doi.org/10.1016/j.colsurfb.2016.04.027.
Acknowledgements
All the authors are thankful to Bhagyoday Tirth Pharmacy College, Sagar, Madhya Pradesh, for providing laboratory facilities, and Dr. H.S. Gour Central University, Sagar, Madhya Pradesh, for TEM facility.
Funding
This study was supported by a Junior Research fellowship from the AICTE, New Delhi.
Author information
Authors and Affiliations
Contributions
KS, SKP: experimental work, drafting.
AM: designing of experiments and performing CLSM studies, in vivo studies, drafting, editing.
RJ: in vivo pharmacodynamic studies, designing of experiments, editing and proof reading before submission.
AJ: conceptu; guidance in experiments, drafting, editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajpoot, K., Prajapati, S.K., Malaiya, A. et al. Meropenem-Loaded Nanostructured Lipid Carriers For Skin and Soft Tissue Infection Caused by Staphylococcus aureus: Formulation, Design, and Evaluation. AAPS PharmSciTech 23, 241 (2022). https://doi.org/10.1208/s12249-022-02381-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02381-y