Abstract
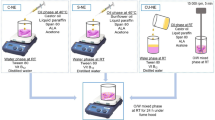
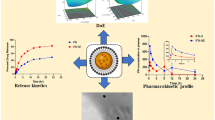
Many active pharmaceutical ingredients (API) are poorly soluble in water and their low oral bioavailability is a major hindrance to their potential use. Megestrol acetate (MGA) is insoluble in water and its oral absorption is limited and considerably affected by food. Nanoemulsions (NEs) can be used as effective oral drug delivery systems where the hydrophobic API is loaded into the oil phase. In this study, MGA-loaded NEs were prepared based on the spontaneous emulsification technique. The effects of different excipients such as ethanol, Tween 80, Lipoid E80, and medium-chain triglyceride (MCT) on the NEs characterization were investigated. The experimental results indicated that optimum MGA-loaded NEs (F20) were nanometer-sized droplets (166.9 ± 3.0 nm) with negative zeta potential (−12.2 ± 1.1 mV). The effect of polyvinylpyrrolidone (PVP) on characteristic properties of F20 was also evaluated. On the selected NEs, in vitro dissolution tests and stability studies in various mediums and storage conditions were performed. The encapsulation efficiency of NEs were > 99%. The overall droplet size of F20 and PVP-2 (PVP-coated NEs) remained relatively stable as the pH changed from 1.2 to 6.8. It was determined that F20 and PVP-2 remained stable at 4°C until 12 weeks and had higher cytotoxicity on MCF-7 cells. To conclude, droplet size, surface charge, and stability are important properties for NEs to have sufficient effectiveness. In this study, alternative oral NEs of low-solubility drug MGA were developed considering the above features.
Graphical Abstract







Similar content being viewed by others
References
Cho E, Cho W, Cha KH, Park J, Kim MS, Kim JS, et al. Enhanced dissolution of megestrol acetate microcrystals prepared by antisolvent precipitation process using hydrophilic additives. Int J Pharmaceut. 2010;396(1-2):91–8. https://doi.org/10.1016/j.ijpharm.2010.06.016.
Ha ES, Kim JS, Baek IH, Yoo JW, Jung YJ, Moon HR, et al. Development of megestrol acetate solid dispersion nanoparticles for enhanced oral delivery by using a supercritical antisolvent process. Drug Des Dev Ther. 2015;9:4269. https://doi.org/10.2147/Dddt.S90706.
Femia RA, Goyette RE. The science of megestrol acetate delivery: potential to improve outcomes in cachexia. BioDrugs. 2005;19(3):179–87. https://doi.org/10.2165/00063030-200519030-00004.
Sylvestre JP, Tang MC, Furtos A, Leclair G, Meunier M, Leroux JC. Nanonization of megestrol acetate by laser fragmentation in aqueous milieu. J Control Release. 2011;149(3):273–80. https://doi.org/10.1016/j.jconrel.2010.10.034.
Alakhov V, Pietrzynski G, Patel K, Kabanov A, Bromberg L, Hatton TA. Pluronic block copolymers and Pluronic poly(acrylic acid) microgels in oral delivery of megestrol acetate. J Pharm Pharmacol. 2004;56(10):1233–41. https://doi.org/10.1211/0022357044427.
Li Y, Song CK, Kim MK, Lim H, Shen Q, Lee DH, et al. Nanomemulsion of megestrol acetate for improved oral bioavailability and reduced food effect. Arch Pharm Res. 2015;38(10):1850–6. https://doi.org/10.1007/s12272-015-0604-9.
Kotta S, Khan AW, Pramod K, Ansari SH, Sharma RK, Ali J. Exploring oral nanoemulsions for bioavailability enhancement of poorly water-soluble drugs. Expert Opin Drug Deliv. 2012;9(5):585–98. https://doi.org/10.1517/17425247.2012.668523.
Shakeel F, Baboota S, Ahuja A, Ali J, Faisal M, Shafiq S. Stability evaluation of celecoxib nanoemulsion containing Tween 80. Thai J Pharm Sci. 2008;32:4–9.
Mehmood T, Ahmed A. Tween 80 and soya-lecithin-based food-grade nanoemulsions for the effective delivery of vitamin D. Langmuir. 2020;36(11):2886–92.
Prabhakar K, Afzal SM, Surender G, Kishan V. Tween 80 containing lipid nanoemulsions for delivery of indinavir to brain. Acta Pharm Sin B. 2013;3(5):345–53.
Kurakula M, Rao G. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): as excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J Drug Deliv Sci Technol. 2020;60:102046. https://doi.org/10.1016/j.jddst.2020.102046.
Yukuyama MN, Ghisleni DDM, Pinto TJA, Bou-Chacra NA. Nanoemulsion: process selection and application in cosmetics - a review. Int J Cosmet Sci. 2016;38(1):13–24. https://doi.org/10.1111/ics.12260.
Liew SN, Utra U, Alias AK, Tan TB, Tan CP, Yussof NS. Physical, morphological and antibacterial properties of lime essential oil nanoemulsions prepared via spontaneous emulsification method. LWT. 2020;128:109388.
Guttoff M, Saberi AH, McClements DJ. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: factors affecting particle size and stability. Food Chem. 2015;171:117–22.
Saberi AH, Fang Y, McClements DJ. Fabrication of vitamin E-enriched nanoemulsions by spontaneous emulsification: effect of propylene glycol and ethanol on formation, stability, and properties. Int Food Res J. 2013;54(1):812–20. https://doi.org/10.1016/j.foodres.2013.08.028.
Ryu V, Corradini MG, McClements DJ, McLandsborough L. Impact of ripening inhibitors on molecular transport of antimicrobial components from essential oil nanoemulsions. J Colloid Interface Sci. 2019;556:568–76.
Nikolic I, Mitsou E, Pantelic I, Randjelovic D, Markovic B, Papadimitriou V, et al. Microstructure and biopharmaceutical performances of curcumin-loaded low-energy nanoemulsions containing eucalyptol and pinene: terpenes’ role overcome penetration enhancement effect? Eur J Pharm Sci. 2020;142:105135.
Lima TS, Silva MFS, Nunes XP, Colombo AV, Oliveira HP, Goto PL, et al. Cineole-containing nanoemulsion: development, stability, and antibacterial activity. Chem Phys Lipids. 2021;239:105113.
García-Márquez E, Higuera-Ciapara I, Espinosa-Andrews H. Design of fish oil-in-water nanoemulsion by microfluidization. Innov Food Sci Emerg Technol. 2017;40:87–91. https://doi.org/10.1016/j.ifset.2016.11.007.
Nemitz MC, von Poser GL, Teixeira HF. In vitro skin permeation/retention of daidzein, genistein and glycitein from a soybean isoflavone rich fraction-loaded nanoemulsions and derived hydrogels. J Drug Deliv Sci Tec. 2019;51:63–9. https://doi.org/10.1016/j.jddst.2019.02.034.
Panatieri LF, Brazil NT, Faber K, Medeiros-Neves B, von Poser GL, Rott MB, et al. Nanoemulsions containing a coumarin-rich extract from Pterocaulon balansae (Asteraceae) for the treatment of ocular Acanthamoeba keratitis. AAPS PharmSciTech. 2017;18(3):721–8. https://doi.org/10.1208/s12249-016-0550-y.
Yalcin TE, Ilbasmis-Tamer S, Ibisoglu B, Özdemir A, Ark M, Takka S. Gemcitabine hydrochloride-loaded liposomes and nanoparticles: comparison of encapsulation efficiency, drug release, particle size, and cytotoxicity. Pharm Dev Technol. 2018;23(1):76–86. https://doi.org/10.1080/10837450.2017.1357733.
Yalcin TE, Yetgin C, Yilmaz A. Development of 5-fluorouracil-loaded nano-sized liposomal formulation by two methods: strategies to enhance encapsulation efficiency of hydrophilic drugs. J Res Pharm. 2021;25(4):371–8. https://doi.org/10.29228/jrp.27.
Talegaonkar S, Mustafa G, Akhter S, Iqbal ZI. Design and development of oral oil-in-water nanoemulsion formulation bearing atorvastatin: in vitro assessment. J Disper Sci Technol. 2010;31(5):690–701. https://doi.org/10.1080/01932690903120540.
Elzoghby AO, Mostafa SK, Helmy MW, ElDemellawy MA, Sheweita SA. Multi-reservoir phospholipid shell encapsulating protamine nanocapsules for co-delivery of letrozole and celecoxib in breast cancer therapy. Pharm Res. 2017;34(9):1956–69. https://doi.org/10.1007/s11095-017-2207-2.
Halder S, Suzuki H, Seto Y, Sato H, Onoue S. Megestrol acetate-loaded self-micellizing solid dispersion system for improved oral absorption and reduced food effect. J Drug Deliv Sci Tec. 2019;49:586–93. https://doi.org/10.1016/j.jddst.2018.12.033.
Feng S-M, Zhao Y, Xu Q, Li H-M, Huang Y-X, Liu H-H, et al. Development and characterization of a new dimethicone nanoemulsion and its application for electronic gastroscopy examination. Int J Nanomed. 2020;15:5405.
Salama AH, Basha M, El Awdan S. Experimentally designed lyophilized dry emulsion tablets for enhancing the antihyperlipidemic activity of atorvastatin calcium: preparation, in-vitro evaluation and in-vivo assessment. Eur J Pharm Sci. 2018;112:52–62. https://doi.org/10.1016/j.ejps.2017.11.003.
Tugcu-Demiroz F, Saar S, Kara AA, Yildiz A, Tuncel E, Acarturk F. Development and characterization of chitosan nanoparticles loaded nanofiber hybrid system for vaginal controlled release of benzydamine. Eur J Pharm Sci. 2021;161:105801. https://doi.org/10.1016/j.ejps.2021.105801.
Chen D, Xia DN, Li XY, Zhu QL, Yu HZ, Zhu CL, et al. Comparative study of Pluronic (R) F127-modified liposomes and chitosan-modified liposomes for mucus penetration and oral absorption of cyclosporine A in rats. Int J Pharmaceut. 2013;449(1-2):1–9. https://doi.org/10.1016/j.ijpharm.2013.04.002.
Şeker Karatoprak G, Yücel Aşık Ç, Çakır A, Köngül ŞE. In vitro pharmacological screening of antioxidant, cytotoxic and enzyme inhibitory activities of Citrus aurantifolia Linn. Dried fruit extract. Int J Environ Health Res. 2021;31(8):991–1000.
Hu Q, Gerhard H, Upadhyaya I, Venkitanarayanan K, Luo Y. Antimicrobial eugenol nanoemulsion prepared by gum arabic and lecithin and evaluation of drying technologies. Int J Biol Macromol. 2016;87:130–40. https://doi.org/10.1016/j.ijbiomac.2016.02.051.
Liu W, Sun D, Li C, Liu Q, Xu J. Formation and stability of paraffin oil-in-water nano-emulsions prepared by the emulsion inversion point method. J Colloid Interface Sci. 2006;303(2):557–63. https://doi.org/10.1016/j.jcis.2006.07.055.
Kim S-H, Ji Y-S, Lee E-S, Hong S-T. Ostwald ripening stability of curcumin-loaded MCT nanoemulsion: influence of various emulsifiers. Prev Nutr Food Sci. 2016;21(3):289–95. https://doi.org/10.3746/pnf.2016.21.3.289.
Zeeb B, Gibis M, Fischer L, Weiss J. Influence of interfacial properties on Ostwald ripening in crosslinked multilayered oil-in-water emulsions. J Colloid Interface Sci. 2012;387(1):65–73. https://doi.org/10.1016/j.jcis.2012.07.054.
Clegg ME. Medium-chain triglycerides are advantageous in promoting weight loss although not beneficial to exercise performance. Int J Food Sci Nutr. 2010;61(7):653–79. https://doi.org/10.3109/09637481003702114.
Llinares R, Santos J, Trujillo-Cayado LA, Ramírez P, Muñoz J. Enhancing rosemary oil-in-water microfluidized nanoemulsion properties through formulation optimization by response surface methodology. LWT. 2018;97:370–5. https://doi.org/10.1016/j.lwt.2018.07.033.
Yang HJ, Cho WG, Park SN. Stability of oil-in-water nano-emulsions prepared using the phase inversion composition method. J Ind Eng Chem. 2009;15(3):331–5. https://doi.org/10.1016/j.jiec.2009.01.001.
Fraga M, Laux M, Zandoná B, Santos G, dos Santos GC, De Oliveira M, et al. Optimization of stearylamine-based nanoemulsions obtained by spontaneous emulsification process as nucleic acids delivery systems. J Drug Deliv Sci Technol. 2008;18(6):398–403.
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57.
Scholz P, Keck CM. Nanoemulsions produced by rotor–stator high speed stirring. Int J Pharm. 2015;482(1):110–7. https://doi.org/10.1016/j.ijpharm.2014.12.040.
Coelho D, Veleirinho B, Mazzarino L, Alberti T, Buzanello E, Oliveira RE, et al. Polyvinyl alcohol-based electrospun matrix as a delivery system for nanoemulsion containing chalcone against Leishmania (Leishmania) amazonensis. Colloids Surf B Biointerfaces. 2021;198:111390.
Gharibzahedi SMT, Mohammadnabi S. Effect of novel bioactive edible coatings based on jujube gum and nettle oil-loaded nanoemulsions on the shelf-life of Beluga sturgeon fillets. Int J Biol Macromol. 2017;95:769–77.
Gomes A, Costa ALR, Cunha RL. Impact of oil type and WPI/Tween 80 ratio at the oil-water interface: adsorption, interfacial rheology and emulsion features. Colloid Surface B. 2018;164:272–80. https://doi.org/10.1016/j.colsurfb.2018.01.032.
Khudair N, Agouni A, Elrayess MA, Najlah M, Younes HM, Elhissi A. Letrozole-loaded nonionic surfactant vesicles prepared via a slurry-based proniosome technology: formulation development and characterization. J Drug Deliv Sci Tec. 2020;58:101721. https://doi.org/10.1016/j.jddst.2020.101721.
Kheradmandnia S, Vasheghani-Farahani E, Nosrati M, Atyabi F. Preparation and characterization of ketoprofen-loaded solid lipid nanoparticles made from beeswax and carnauba wax. Nanomed J. 2010;6(6):753–9. https://doi.org/10.1016/j.nano.2010.06.003.
Witayaudom P, Klinkesorn U. Effect of surfactant concentration and solidification temperature on the characteristics and stability of nanostructured lipid carrier (NLC) prepared from rambutan (Nephelium lappaceum L.) kernel fat. J Colloid Interface Sci. 2017;505:1082–92. https://doi.org/10.1016/j.jcis.2017.07.008.
Teeranachaideekul V, Chantaburanan T, Junyaprasert VB. Influence of state and crystallinity of lipid matrix on physicochemical properties and permeation of capsaicin-loaded lipid nanoparticles for topical delivery. J Drug Deliv Sci Tec. 2017;39:300–7. https://doi.org/10.1016/j.jddst.2017.04.003.
Kriegel C, Festag M, Kishore RSK, Roethlisberger D, Schmitt G. Pediatric safety of polysorbates in drug formulations. Children (Basel). 2019;7(1):1. https://doi.org/10.3390/children7010001.
Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49. https://doi.org/10.1016/j.jconrel.2017.03.008.
Uluata S, Decker EA, McClements DJ. Optimization of nanoemulsion fabrication using microfluidization: role of surfactant concentration on formation and stability. Food Biophys. 2016;11(1):52–9. https://doi.org/10.1007/s11483-015-9416-1.
Riquelme N, Zuniga RN, Arancibia C. Physical stability of nanoemulsions with emulsifier mixtures: replacement of Tween 80 with Quillaja saponin. LWT. 2019;111:760–6. https://doi.org/10.1016/j.lwt.2019.05.067.
Iqbal R, Mehmood Z, Baig A, Khalid N. Formulation and characterization of food grade O/W nanoemulsions encapsulating quercetin and curcumin: Insights on enhancing solubility characteristics. Food Bioprod Process. 2020;123:304–11. https://doi.org/10.1016/j.fbp.2020.07.013.
Galindo-Alvarez J, Sadtler V, Marchal P, Perrin P, Tribet C, Marie E, et al. Nanoemulsions with enhanced temperature stability using thermo-sensitive association of nonionic surfactant and amphiphilic polyelectrolytes. Colloid Surface A. 2012;396:115–21. https://doi.org/10.1016/j.colsurfa.2011.12.051.
Esquerdo VM, Silva PP, Dotto GL, Pinto LAA. Nanoemulsions from unsaturated fatty acids concentrates of carp oil using chitosan, gelatin, and their blends as wall materials. Eur J Lipid Sci Tech. 2018;120(2):1700240. https://doi.org/10.1002/ejlt.201700240.
Bai YF, Wang D, Zhang Z, Pan JC, Cui ZB, Yu DG, et al. Testing of fast dissolution of ibuprofen from its electrospun hydrophilic polymer nanocomposites. Polym Test. 2021;93:106872. https://doi.org/10.1016/j.polymertesting.2020.106872.
Pavlovic M, Antonietti M, Schmidt B, Zeininger L. Responsive Janus and Cerberus emulsions via temperature-induced phase separation in aqueous polymer mixtures. J Colloid Interface Sci. 2020;575:88–95. https://doi.org/10.1016/j.jcis.2020.04.067.
Hadzir NM, Basri M, Rahman MBA, Salleh A, Rahman RNZRA, Basri H. Phase behaviour and formation of fatty acid esters nanoemulsions containing piroxicam. AAPS PharmSciTech. 2013;14(1):456–63. https://doi.org/10.1208/s12249-013-9929-1.
Ghosh S, Coupland JN. Factors affecting the freeze–thaw stability of emulsions. Food Hydrocolloids. 2008;22(1):105–11.
Kori A, Mahesar S, Sherazi S, Khatri U, Laghari Z, Panhwar T. Effect of process parameters on emulsion stability and droplet size of pomegranate oil-in-water. Grasas y Aceites. 2021;72(2):e410-e.
Yang J, Gu Z, Cheng L, Li Z, Li C, Hong Y. Effect of temperature, pH, and ionic strength on the structure and physical stability of double emulsions prepared with starch. LWT. 2021;141:111086.
Ganta S, Sharma P, Paxton JW, Baguley BC, Garg S. Pharmacokinetics and pharmacodynamics of chlorambucil delivered in long-circulating nanoemulsion. J Drug Target. 2010;18(2):125–33. https://doi.org/10.3109/10611860903244199.
Elsheikh MA, Elnaggar YS, Gohar EY, Abdallah OY. Nanoemulsion liquid preconcentrates for raloxifene hydrochloride: optimization and in vivo appraisal. Int J Nanomed. 2012;7:3787–802. https://doi.org/10.2147/IJN.S33186.
Montenegro L, Carbone C, Condorelli G, Drago R, Puglisi G. Effect of oil phase lipophilicity on in vitro drug release from o/w microemulsions with low surfactant content. Drug Dev Ind Pharm. 2006;32(5):539–48.
Seo YD, Jin SE, Kim D, Lee DH, Yang SG. Fabrication of Eudragit polymeric nanoparticles using ultrasonic nebulization method for enhanced oral absorption of megestrol acetate. Pharm Dev Technol. 2018;23(4):407–13. https://doi.org/10.1080/10837450.2017.1400049.
Zu Y, Sun W, Zhao X, Wang W, Li Y, Ge Y, et al. Preparation and characterization of amorphous amphotericin B nanoparticles for oral administration through liquid antisolvent precipitation. Eur J Pharm Sci. 2014;53:109–17. https://doi.org/10.1016/j.ejps.2013.12.005.
Patel GV, Patel VB, Pathak A, Rajput SJ. Nanosuspension of efavirenz for improved oral bioavailability: formulation optimization, in vitro, in situ and in vivo evaluation. Drug Dev Ind Pharm. 2014;40(1):80–91. https://doi.org/10.3109/03639045.2012.746362.
Ravi M, Bhavani S, Kumar KK, Rao VVRN. Investigations on electrical properties of PVP:KIO4 polymer electrolyte films. Solid State Sci. 2013;19:85–93. https://doi.org/10.1016/j.solidstatesciences.2013.02.006.
Aditya NP, Macedo AS, Doktorovov S, Souto EB, Kim S, Chang PS, et al. Development and evaluation of lipid nanocarriers for quercetin delivery: a comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE). LWT. 2014;59(1):115–21. https://doi.org/10.1016/j.lwt.2014.04.058.
Liu Y, Yang T, Wei S, Zhou C, Lan Y, Cao A, et al. Mucus adhesion- and penetration-enhanced liposomes for paclitaxel oral delivery. Int J Pharm. 2018;537(1-2):245–56. https://doi.org/10.1016/j.ijpharm.2017.12.044.
Liu Q, Cheng J, Sun X, Guo M. Preparation, characterization, and antioxidant activity of zein nanoparticles stabilized by whey protein nanofibrils. Int J Biol Macromol. 2021;167:862–70. https://doi.org/10.1016/j.ijbiomac.2020.11.043.
Caddeo C, Pons R, Carbone C, Fernandez-Busquets X, Cardia MC, Maccioni AM, et al. Physico-chemical characterization of succinyl chitosan-stabilized liposomes for the oral co-delivery of quercetin and resveratrol. Carbohydr Polym. 2017;157:1853–61. https://doi.org/10.1016/j.carbpol.2016.11.072.
Khan MS, Bhat SA, Rehman MT, Hassan I, Tabrez S, AlAjmi MF, et al. Rutin attenuates negatively charged surfactant (SDS)-induced lysozyme aggregation/amyloid formation and its cytotoxicity. Int J Biol Macromol. 2018;120(Pt A):45–58. https://doi.org/10.1016/j.ijbiomac.2018.07.112.
Kelmann RG, Kuminek G, Teixeira HF, Koester LS. Carbamazepine parenteral nanoemulsions prepared by spontaneous emulsification process. Int J Pharm. 2007;342(1-2):231–9. https://doi.org/10.1016/j.ijpharm.2007.05.004.
Shilpa JB, Sheeba GM. Automated real time monitoring for food grain storage. Int J Pure Appl Math. 2018;118(24):1314–3395.
Li PH, Lu WC. Effects of storage conditions on the physical stability of D-limonene nanoemulsion. Food Hydrocolloid. 2016;53:218–24. https://doi.org/10.1016/j.foodhyd.2015.01.031.
Machado FC, de Matos RPA, Primo FL, Tedesco AC, Rahal P, Calmon MF. Effect of curcumin-nanoemulsion associated with photodynamic therapy in breast adenocarcinoma cell line. Bioorg Med Chem. 2019;27(9):1882–90.
Ma HL, Varanda LC, Perussi JR, Carrilho E. Hypericin-loaded oil-in-water nanoemulsion synthesized by ultrasonication process enhances photodynamic therapy efficiency. J Photochem Photobiol B Biol. 2021;223:112303.
Badr-Eldin SM, Aldawsari HM, Ahmed OAA, Alhakamy NA, Neamatallah T, Okbazghi SZ, et al. Optimized semisolid self-nanoemulsifying system based on glyceryl behenate: a potential nanoplatform for enhancing antitumor activity of raloxifene hydrochloride in MCF-7 human breast cancer cells. Int J Pharm. 2021;600:120493. https://doi.org/10.1016/j.ijpharm.2021.120493.
Srivastava S, Singh S, Saraf SA, Chourasia MK, Mathew J, Pandey AC. Encapsulation of baicalein in cinnamon essential oil nanoemulsion for enhanced anticancer efficacy against MDA-MB-231 cells. BioNanoScience. 2021;11(4):1049–60.
Raviadaran R, Ng MH, Chandran D, Ooi KK, Manickam S. Stable W/O/W multiple nanoemulsion encapsulating natural tocotrienols and caffeic acid with cisplatin synergistically treated cancer cell lines (A549 and HEP G2) and reduced toxicity on normal cell line (HEK 293). Mater Sci Eng C. 2021;121:111808.
Acknowledgements
The authors want to thank Deva Holding AS for supplying MGA and Lipoid GmbH for supplying egg lecithin (Lipoid E80). The authors are also grateful to Prof. Didem DELIORMAN ORHAN and Prof. Esra KUPELI AKKOL (Gazi University Faculty of Pharmacy, Department of Pharmacognosy) for providing some facilities to carry out this research.
Author information
Authors and Affiliations
Contributions
Tahir E. Yalcin: conceptualization, investigation, methodology, formal analysis, writing—original draft. Emre Tuncel: conceptualization, investigation, methodology, formal analysis, writing—original draft. Cigdem Yucel: conceptualization, investigation, methodology, formal analysis, writing—original draft of cytotoxicity section. Figen Tirnaksiz: conceptualization, methodology, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 286 kb)
Rights and permissions
About this article
Cite this article
Yalcin, T.E., Tuncel, E., Yucel, C. et al. Nanoemulsions Containing Megestrol Acetate: Development, Characterization, and Stability Evaluation. AAPS PharmSciTech 23, 142 (2022). https://doi.org/10.1208/s12249-022-02289-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02289-7