Abstract
Arsenic trioxide (ATO) has efficient anticancer effect on hepatocellular carcinoma (HCC) in clinical trials, but its off-target distribution and side effects have limited its use. Here, we demonstrate an albumin-embellished ATO-loaded polyethylene glycol–polycaprolactone–polyethyleneimine (PEG–PCL–PEI) nanoparticle (AATONP) to enhance the tumor distribution and intratumor drug release of ATO for HCC therapy. AATONP is prepared by surface embellishment with albumin on the cationic ATO-loaded PEG–PCL–PEI nanoparticles (CATONP). Albumin embellishment can reduce the cationic material’s hemolytic toxicity in blood cells while maintaining the rapid internalization and lysosome escape abilities of the positively charged CATONP. AATONP provides sustained and low pH-responsive drug release, facilitating the targeted drug release in the intratumor acidic microenvironment. Moreover, AATONP can significantly improve the circulation time and tumor distribution of ATO via albumin-mediated transcytosis in HCC tumor-bearing mice. Compared with free ATO and the clinically used nanomedicine Genexol/PM, AATONP shows potent antitumor activity against a human HCC xenograft mouse model, leading to a higher tumor inhibition rate of 89.4% in HCC therapy. In conclusion, this work presents an efficient strategy to achieve tumor accumulation and the intratumor drug release of ATO for HCC therapy.
Graphical abstract
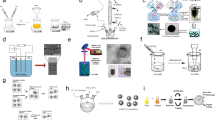
An albumin-embellished arsenic trioxide (ATO)-loaded polyethylene glycol–polycaprolactone–polyethyleneimine nanoparticle (AATONP) is designed to enhance tumor distribution and intratumor drug release of ATO for hepatocellular carcinoma therapy. AATONP can achieve enhanced tumor distribution via albumin-mediated transcytosis and exhibit intratumor drug release of ATO via tumor acidic microenvironment-response, leading to potent antitumor activity.









Similar content being viewed by others
References
Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111(5):2505–15. https://doi.org/10.1182/blood-2007-07-102798.
Park J, Jurcic JG, Rosenblat T, Tallman MS. Emerging new approaches for the treatment of acute promyelocytic leukemia. Ther Adv Hematol. 2011;2(5):335–52. https://doi.org/10.1177/2040620711410773.
Fei W, Li C, Tao J, Cai X, Yao W, Ye Y, Zhang Y, Yao Y, Song Q, Li F, Zheng C. Construction of arsenic-metal complexes loaded nanodrugs for solid tumor therapy: A mini review. Int J Pharm. 2020;583:119385. https://doi.org/10.1016/j.ijpharm.2020.119385.
Akhtar A, Wang SX, Ghali L, Bell C, Wen X. Recent advances in arsenic trioxide encapsulated nanoparticles as drug delivery agents to solid cancers. J Biomed Res. 2017;31(3):177–88. https://doi.org/10.7555/jbr.31.20160059.
Zhang L, Zhou Y, Kong J, Zhang L, Yuan M, Xian S, Wang Y, Cheng Y, Yang X. Effect of arsenic trioxide on cervical cancer and its mechanisms. Exp Ther Med. 2020;20(6):169–72. https://doi.org/10.3892/etm.2020.9299.
Tao J, Fei W, Tang H, Li C, Mu C, Zheng H, Li F, Zhu Z. Angiopep-2-Conjugated "Core-Shell" Hybrid Nanovehicles for Targeted and pH-Triggered Delivery of Arsenic Trioxide into Glioma. Mol Pharm. 2019;16(2):786–97. https://doi.org/10.1021/acs.molpharmaceut.8b01056.
Lu Y, Han S, Zheng H, Ma R, Ping Y, Zou J, Tang H, Zhang Y, Xu X, Li F. A novel RGDyC/PEG co-modified PAMAM dendrimer-loaded arsenic trioxide of glioma targeting delivery system. Int J Nanomedicine. 2018;13:5937–52. https://doi.org/10.2147/ijn.S175418.
Fei W, Zhang Y, Han S, Tao J, Zheng H, Wei Y, Zhu J, Li F, Wang X. RGD conjugated liposome-hollow silica hybrid nanovehicles for targeted and controlled delivery of arsenic trioxide against hepatic carcinoma. Int J Pharm. 2017;519(1-2):250–62. https://doi.org/10.1016/j.ijpharm.2017.01.031.
Zheng T, Yin D, Lu Z, Wang J, Li Y, Chen X, Liang Y, Song X, Qi S, Sun B, Xie C, Meng X, Pan S, Liu J, Jiang H, Liu L. Nutlin-3 overcomes arsenic trioxide resistance and tumor metastasis mediated by mutant p53 in Hepatocellular Carcinoma. Mol Cancer. 2014;13(1):133. https://doi.org/10.1186/1476-4598-13-133.
Shooshtary S, Behtash S, Nafisi S. Arsenic trioxide binding to serum proteins. J Photochem Photobiol B Biol. 2015;148:31–6. https://doi.org/10.1016/j.jphotobiol.2015.03.001.
Swindell EP, Hankins PL, Chen H, Miodragovic DU, O'Halloran TV. Anticancer Activity of Small-Molecule and Nanoparticulate Arsenic(III) Complexes. Inorg Chem. 2013;52(21):12292–304. https://doi.org/10.1021/ic401211u.
Song X, You J, Wang J, Zhu A, Ji L, Guo R. Preparation and Investigation of Arsenic Trioxide-loaded Polylactic Acid/Magnetic Hybrid Nanoparticles. Chem Res Chin Univ. 2014;30(2):326–32. https://doi.org/10.1007/s40242-014-3306-9.
Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. https://doi.org/10.1038/nrc.2016.108.
Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv Drug Deliv Rev. 2017;108:25–38. https://doi.org/10.1016/j.addr.2016.04.025.
He L, Liu Y, Lau J, Fan W, Li Q, Zhang C, Huang P, Chen X. Recent progress in nanoscale metal-organic frameworks for drug release and cancer therapy. Nanomedicine. 2019;14(10):1343–65. https://doi.org/10.2217/nnm-2018-0347.
Hoonjan M, Jadhav V, Bhatt P. Arsenic trioxide: insights into its evolution to an anticancer agent. J Biol Inorg Chem. 2018;23(3):313–29. https://doi.org/10.1007/s00775-018-1537-9.
Xiao X, Liu Y, Guo M, Fei W, Zheng H, Zhang R, Zhang Y, Wei Y, Zheng G, Li F. pH-triggered sustained release of arsenic trioxide by polyacrylic acid capped mesoporous silica nanoparticles for solid tumor treatment in vitro and in vivo. J Biomater Appl. 2016;31(1):23–35. https://doi.org/10.1177/0885328216637211.
Croissant JG, Fatieiev Y, Khashab NM. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv Mater. 2017;29(9):1604634. https://doi.org/10.1002/adma.201604634.
He Q, Zhang Z, Gao Y, Shi J, Li Y. Intracellular Localization and Cytotoxicity of Spherical Mesoporous Silica Nano- and Microparticles. Small. 2009;5(23):2722–9. https://doi.org/10.1002/smll.200900923.
Laechelt U, Wagner E. Nucleic Acid Therapeutics Using Polyplexes: A Journey of 50 Years (and Beyond). Chem Rev. 2015;115(19):11043–78. https://doi.org/10.1021/cr5006793.
Chen D, Han S, Zhu Y, Hu F, Wei Y, Wang G. Kidney-targeted drug delivery via rhein-loaded polyethyleneglycol-co-polycaprolactone-co-polyethylenimine nanoparticles for diabetic nephropathy therapy. Int J Nanomedicine. 2018;13:3507–27. https://doi.org/10.2147/ijn.S166445.
Robb R, Kuo JC-T, Liu Y, Corrales-Guerrero S, Cui T, Hegazi A, Nagy G, Lee RJ, Williams TM. A novel protein-drug conjugate, SSH20, demonstrates significant efficacy in caveolin-1-expressing tumors. Mol Ther-Oncol. 2021;22:555–64. https://doi.org/10.1016/j.omto.2021.07.013.
Hoogenboezem EN, Duvall CL. Harnessing albumin as a carrier for cancer therapies. Adv Drug Deliv Rev. 2018;130:73–89. https://doi.org/10.1016/j.addr.2018.07.011.
Hama M, Ishima Y, Chuang VTG, Ando H, Shimizu T, Ishida T. Evidence for Delivery of Abraxane via a Denatured-Albumin Transport System. ACS Appl Mater Interfaces. 2021;13(17):19736–44. https://doi.org/10.1021/acsami.1c03065.
Wang G, Zhou Z, Zhao Z, Li Q, Wu Y, Yan S, Shen Y, Huang P. Enzyme-Triggered Transcytosis of Dendrimer-Drug Conjugate for Deep Penetration into Pancreatic Tumors. ACS Nano. 2020;14(4):4890–904. https://doi.org/10.1021/acsnano.0c00974.
Zheng X, Xie J, Zhang X, Sun W, Zhao H, Li Y, Wang C. An overview of polymeric nanomicelles in clinical trials and on the market. Chin Chem Lett. 2020;32(1):243–57. https://doi.org/10.1016/j.cclet.2020.11.029.
Sun XR, Wang GW, Zhang H, Hu SQ, Liu X, Tang JB, Shen YQ. The Blood Clearance Kinetics and Pathway of Polymeric Micelles in Cancer Drug Delivery. ACS Nano. 2018;12(6):6179–92. https://doi.org/10.1021/acsnano.8b02830.
Sun Q, Zhou Z, Qiu N, Shen Y. Rational Design of Cancer Nanomedicine: Nanoproperty Integration and Synchronization. Adv Mater. 2017;29(14):1606628. https://doi.org/10.1002/adma.201606628.
Wang HX, Zuo ZQ, Du JZ, Wang YC, Sun R, Cao ZT, Ye XD, Wang JL, Leong KW, Wang J. Surface charge critically affects tumor penetration and therapeutic efficacy of cancer nanomedicines. Nano Today. 2016;11(2):133–44. https://doi.org/10.1016/j.nantod.2016.04.008.
Manaargadoo-Catin M, Ali-Cherif A, Pougnas J-L, Perrin C. Hemolysis by surfactants-A review. Adv Colloid Interf Sci. 2016;228:1–16. https://doi.org/10.1016/j.cis.2015.10.011.
Tsoi KM, MacParland SA, Ma X-Z, Spetzler VN, Echeverri J, Ouyang B, Fadel SM, Sykes EA, Goldaracena N, Kaths JM, Conneely JB, Alman BA, Selzner M, Ostrowski MA, Adeyi OA, Zilman A, McGilvray ID, Chan WCW. Mechanism of hard-nanomaterial clearance by the liver. Nat Mater. 2016;15(11):1212–21. https://doi.org/10.1038/nmat4718.
Wang G, Bihan W, Li Q, Chen S, Jin X, Liu Y, Zhou Z, Shen Y, Huang P. Active transportation of liposome enhances tumor accumulation, penetration, and therapeutic efficacy. Small. 2020;16:2004172. https://doi.org/10.1002/smll.202004172.
Winter N, Murphy RJ, o Halloran T, Schatz G. Development and modeling of arsenic-trioxide loaded thermosensitive liposomes for anticancer drug delivery. Journal of Liposome. Research. 2011;21:106–15. https://doi.org/10.3109/08982104.2010.483597.
Kanamala M, Wilson WR, Yang M, Palmer BD, Wu Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials. 2016;85:152–67. https://doi.org/10.1016/j.biomaterials.2016.01.061.
Zhang Q, Vakili MR, Li X-F, Lavasanifar A, Le XC. Polymeric micelles for GSH-triggered delivery of arsenic species to cancer cells. Biomaterials. 2014;35(25):7088–100. https://doi.org/10.1016/j.biomaterials.2014.04.072.
Miller WH, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62(14):3893–903.
Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003. https://doi.org/10.1038/nmat3776.
Schoettler S, Becker G, Winzen S, Steinbach T, Mohr K, Landfester K, Mailaender V, Wurm FR. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol. 2016;11(4):372–7. https://doi.org/10.1038/nnano.2015.330.
Su G, Jiang H, Xu B, Yu Y, Chen X. Effects of Protein Corona on Active and Passive Targeting of Cyclic RGD Peptide-Functionalized PEGylation Nanoparticles. Mol Pharm. 2018;15(11):5019–30. https://doi.org/10.1021/acs.molpharmaceut.8b00612.
Acknowledgments
This work was partially supported by the Natural Science Foundation of China (Nos. 82003669, 82102191), Science and Technology Program on Medicine and Health of Zhejiang Province (Nos. 2017KY511, 2022KY913), and Natural Science Foundation of Zhejiang Province (No. LYY21H310001).
Author information
Authors and Affiliations
Contributions
Yu Huang contributed to investigation, data curation, project administration, methodology, and writing original draft. Zhishi Xu contributed to investigation, validation, software, and writing original draft. Yinghui Wei contributed to conceptualization and validation. Shunping Han contributed to software and writing original draft. Xinjun Cai contributed to conceptualization, funding acquisition, and writing review and editing. Danfei Chen contributed to supervision, funding acquisition, conceptualization, project administration, and writing review and editing.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest in this work.
Additional information
Guest Editor. Claudio Salomom
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Theme: Recent Advances on Drug Delivery Systems for Viral Infections
Rights and permissions
About this article
Cite this article
Huang, Y., Xu, Z., Wei, Y. et al. Albumin-Embellished Arsenic Trioxide-Loaded Polymeric Nanoparticles Enhance Tumor Accumulation and Anticancer Efficacy via Transcytosis for Hepatocellular Carcinoma Therapy. AAPS PharmSciTech 23, 111 (2022). https://doi.org/10.1208/s12249-022-02254-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02254-4