Abstract
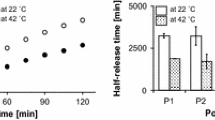
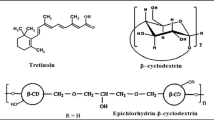
Topical formulation of non-steroidal anti-inflammatory drugs (NSAIDs) exhibits many advantages over the oral administration route, such as avoiding the direct effect on GIT and avoiding the poor oral bioavailability of such drugs. Our study aims to develop a new self-assembling construct based on the hydrophobic interaction between adamantane terminated poly (ethylene glycol) polymers and polymerized β-cyclodextrin. The viscous constructs were developed from direct mixing of host and guest polymer solutions, indicating spontaneous formation without cross-linkers. The modified system was evaluated by different analyses, including X-ray diffractometry, electron microscopy, isothermal titration calorimetry, and rheological analysis. Moreover, such a system’s ability for drug loading and release was investigated via the in vitro release of ketorolac tromethamine (KT) as a model of NSAIDs. Finally, the prepared formulas were applied on a rat paw edema model to prove the enhanced anti-inflammatory activities. The obtained results indicated that the modified constructs have a rubbery porous structure with an amorphous nature. Also, from rheological results, the modified system exhibited a viscous behavior with higher loss modulus (G″) compared with storage (G′). The inclusion complexation between cyclodextrin and adamantane moieties was proved by the recorded high binding constants with a 1:1 stoichiometric ratio. Furthermore, the results showed the successful KT incorporation into the modified system and quantitatively released through a semi-permeable membrane in a sustained fashion (over 24 h). Finally, the in vivo results of the medicated constructs showed a significant inhibition of the induced inflammation and swelling, indicating that the modified construct has a great utility for safe non-irritating topical delivery applications.











Similar content being viewed by others
References
Khurana S, Chhillar N, Gautam VKS. Inventory control techniques in medical stores of a tertiary care neuropsychiatry hospital in Delhi. Health. 2013;05(01):8–13.
Cho YA, Gwak HS. Transdermal delivery of ketorolac tromethamine: effects of vehicles and penetration enhancers. Drug Dev Ind Pharm. 2004;30(6):557–64.
Pabreja K, Dua K, Gorajana A. Evaluation of topical gels containing ketorolac tromethamine on inflammation and hyperalgesia in rats. Anti-Inflamm Anti-Allergy Agents Med Chem. 2012;10(5):323–6.
Brocks DR, Jamali F. Clinical pharmacokinetic of ketorolac tromethamine. Clin Pharmacokinet. 1992;23(6):415–27.
Del Valle EMM. Cyclodextrins and their uses: a review. Process Biochemistry. 2004;39(9):1033–46.
Mojumdar EH, Grey C, Sparr E. Self-assembly in gangliosidephospholipid systems: the co-existence of vesicles, micelles, and discs. Int J Mol Sci. 2019;21(1):56.
Nielsen AL, Steffensen K, Larsen KL. Self-assembling microparticles with controllable disruption properties based on cyclodextrin interactions. Colloids Surf B Biointerfaces. 2009;73(2):267–75.
Renard E. Volet Gl, Amiel C. Synthesis of a novel linear water-soluble β-cyclodextrin polymer. Polymer International. 2005;54(3):594–9.
van de Manakker F, van der Pot M, Vermonden T, van Nostrum CF, Hennink WE. Self-assembling hydrogels based on β-cyclodextrin/cholesterol inclusion complexes. Macromolecules. 2008;41(5):1766–73.
van de Manakker F, Kroon-Batenburg LMJ, Vermonden T, van Nostrum CF, Hennink WE. Supramolecular hydrogels formed by β-cyclodextrin self-association and host–guest inclusion complexes. Soft Matter. 2010;6(1):187–94.
Gref R, Amiel C, Molinard K, Daoud-Mahammed S, Sebille B, Gillet B, et al. New self-assembled nanogels based on host-guest interactions: characterization and drug loading. J Control Release. 2006;111(3):316–24.
Koopmans C, Ritter H. Formation of physical hydrogels via host−guest interactions of β-cyclodextrin polymers and copolymers bearing adamantyl groups. Macromolecules. 2008;41(20):7418–22.
Daoud-Mahammed S, Couvreur P, Bouchemal K, Chéron M, Lebas GV, Amiel C, et al. Cyclodextrin and polysaccharide-based nanogels: entrapment of two hydrophobic molecules, benzophenone and tamoxifen. Biomacromolecules. 2009;10(3):547–54.
Layre AM, Volet G, Wintgens V, Amiel C. Associative network based on cyclodextrin polymer: a model system for drug delivery. Biomacromolecules. 2009;10(12):3283–9.
Zhang J, Ma PX. Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv Drug Deliv Rev. 2013;65(9):1215–33.
Takashima Y, Nakayama T, Miyauchi M, Kawaguchi Y, Yamaguchi H, Harada A. Complex formation and gelation between copolymers containing pendant azobenzene groups and cyclodextrin polymers. Chemistry Letters. 2004;33(7):890–1.
Wang J, Qiu Z, Wang Y, Li L, Guo X, Pham DT, Lincoln SF, Prud’homme RK. Supramolecular polymer assembly in aqueous solution arising from cyclodextrin host-guest complexation. Beilstein J Org Chem. 2016;12:50–72.
Li J, Harada A, Kamachi M. Sol–gel transition during inclusion complex formation between α-cyclodextrin and high molecular weight poly(ethylene glycol)s in aqueous solution. Polymer Journal. 1994;26(9):1019–26.
Li J. Cyclodextrin inclusion polymers forming hydrogels. Inclusion Polymers. Advances in Polymer Science 2009. p. 175-203.
Daoud-Mahammed S, Grossiord JL, Bergua T, Amiel C, Couvreur P, Gref R. Self-assembling cyclodextrin based hydrogels for the sustained delivery of hydrophobic drugs. J Biomed Mater Res A. 2008;86(3):736–48.
Li J. Self-assembled supramolecular hydrogels based on polymer–cyclodextrin inclusion complexes for drug delivery. NPG Asia Materials. 2010;2(3):112–8.
Park H, Park K. Biocompatibility issues of implantable drug delivery systems. Pharm Res. 1996;13(12):1770–6.
Kamath KR, Park K. Biodegradable hydrogels in drug delivery. Adv Drug Deliv Rev. 1993;11(1-2):59–84.
Heller J. Polymers for controlled parenteral delivery of peptides and proteins. Adv Drug Deliv Rev. 1993;10(2-3):163–204.
Auzély-Velty R, Rinaudo M. New supramolecular assemblies of a cyclodextrin-grafted chitosan through specific complexation. Macromolecules. 2002;35(21):7955–62.
Charlot A, Auzély-Velty R, Rinaudo M. Specific interactions in model charged polysaccharide systems†. J Phys Chem B. 2003;107(32):8248–54.
Hatefi A, Amsden B. Biodegradable injectable in situ forming drug delivery systems. J Control Release. 2002;80(1-3):9–28.
Budavari S, O’Neil M, Smith A. The Merck Index. 13th ed. Whitehouse Station, NJ: Merck & Co. Inc; 1996. p. 948.
Harries D, Rau DC, Parsegian VA. Solutes probe hydration in specific association of cyclodextrin and adamantane. J Am Chem Soc. 2005;127(7):2184–90.
Osman SK, Brandl FP, Zayed GM, Teßmar JK, Göpferich AM. Cyclodextrin based hydrogels: inclusion complex formation and micellization of adamantane and cholesterol grafted polymers. Polymer. 2011;52(21):4806–12.
Osman SK, Soliman GM, El Rasoul SA. Physically crosslinked hydrogels of beta -cyclodextrin polymer and poly(ethylene glycol)-cholesterol as delivery systems for macromolecules and small drug molecules. Curr Drug Deliv. 2015;12(4):415–24.
Abdellatif AAH, Ibrahim MA, Amin MA, Maswadeh H, Alwehaibi MN, Al-Harbi SN, et al. Cetuximab conjugated with octreotide and entrapped calcium alginate-beads for targeting somatostatin receptors. Sci Rep. 2020;10(1):4736.
Siewert M, Dressman J, Brown CK, Shah VP, Fip, Aaps. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech. 2003;4(1):E7.
Mohammed AM, Osman SK, Saleh KI, Samy AM. In vitro release of 5-fluorouracil and methotrexate from different thermosensitive chitosan hydrogel systems. AAPS PharmSciTech. 2020;21(4):131.
Laskar PA. Physical pharmacy - physical and chemical principles in the pharmaceutical sciences - Martin, A, Swarbrick, J, Cammarata, A. Am J Pharm Educ. 1984;48(1):107–8.
Wagenmakers EJ. Model selection and multimodel inference: a practical information-theoretic approach. J Math Psychol. 2003;47(5-6):580–6.
Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Exper Biol Med. 1962;111(3):544–7.
Habib FS, Hassan MAA, Abou El Ela AEF, Raheem RESA. Different topical formulations of ketorolac tromethamine for anti-inflammatory application and clinical efficacy. Digest J Nano Biost. 2014;9(2):705–19.
Khunt DM, Mishra AD, Shah DR. Formulation design & development of piroxicam emulgel. Int J Pharm Tech Res. 2012;4(3):1332–44.
Bhanu PV, Shanmugam V, Lakshmi P. Development and optimization of novel diclofenac emulgel for topical drug delivery. Int J Compreh Pharm. 2011;9:1–4.
Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82(3):377–90.
Loh XJ, Colin Sng KB, Li J. Synthesis and water-swelling of thermo-responsive poly(ester urethane)s containing poly(epsilon-caprolactone), poly(ethylene glycol) and poly(propylene glycol). Biomaterials. 2008;29(22):3185–94.
Bandi N, Wei W, Roberts CB, Kotra LP, Kompella UB. Preparation of budesonide- and indomethacin-hydroxypropyl-beta-cyclodextrin(HPBCD) complexes using a single-step, organic-solvent-free supercritical fluid process. Eur J Pharm Sci. 2004;23(2):159–68.
Sant VP, Nagarsenker MS. Synthesis of monomethoxypolyethyleneglycol-cholesteryl ester and effect of its incorporation in liposomes. AAPS PharmSciTech. 2011;12(4):1056–63.
Choi SG, Lee SE, Kang BS, Ng CL, Davaa E, Park JS. Thermosensitive and mucoadhesive sol-gel composites of paclitaxel/dimethyl-beta-cyclodextrin for buccal delivery. PLoS One. 2014;9(9):e109090.
Tronci G, Ajiro H, Russell SJ, Wood DJ, Akashi M. Tunable drug-loading capability of chitosan hydrogels with varied network architectures. Acta Biomater. 2014;10(2):821–30.
Guo B, Finne-Wistrand A, Albertsson AC. Facile synthesis of degradable and electrically conductive polysaccharide hydrogels. Biomacromolecules. 2011;12(7):2601–9.
Zhang H, Peng L, Xin Y, Yan Q, Yuan J. Stimuli-responsive polymer networks with β-cyclodextrin and ferrocene reversible linkage based on linker chemistry. Macromol Symp. 2013;329(1):66–9.
Ritger PL, Peppas NA. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Cont Rel. 1987;5(1):37–42.
Helal DA, El-Rhman DA, Abdel-Halim SA, El-Nabarawi MA. Formulation and evaluation of fluconazole topical gel. Int J Pharm Pharm Sci. 2012;4(5):302–10.
Author information
Authors and Affiliations
Contributions
Conceptualization, Ahmed A. H. Abdellatif and Shaaban K. Osman; data curation, Gamal Zayed; formal analysis, Ahmed M. Mohammed and Shaaban K. Osman; investigation, Ahmed A. H. Abdellatif and Shaaban K. Osman; methodology, Shaaban K. Osman; project administration, Ahmed A. H. Abdellatif, Saleh Abd El-Rasoul, and Shaaban K. Osman; resources, Ahmed A. H. Abdellatif, Gamal Zayed, Saud A. Almawash, and Shaaban K. Osman; software, Ahmed M. Mohammed and Mohamed A. Safwat; validation, Ahmed A. H. Abdellatif and Saleh Abd El-Rasoul; visualization, Saleh Abd El-Rasoul, Saud A. Almawash, Mohamed A. Safwat, and Shaaban K. Osman; writing — original draft, Ahmed M. Mohammed, Gamal Zayed, and Shaaban K. Osman; writing — review and editing, Ahmed A. H. Abdellatif and Shaaban K. Osman.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethical Approval
The procedures were approved by the Animal Ethics Committee of Al-Azhar University, Faculty of Pharmacy, Assiut, Egypt (Approval No. AZ-AS/PH/3/C/2021).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdellatif, A.A.H., Mohammed, A.M., Zayed, G. et al. Cyclodextrin/Adamantane-Grafted Polyethylene Glycol–Based Self-assembling Constructs for Topical Delivery of Ketorolac Tromethamine: Formulation, Characterization, and In Vivo Studies. AAPS PharmSciTech 23, 45 (2022). https://doi.org/10.1208/s12249-021-02188-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02188-3