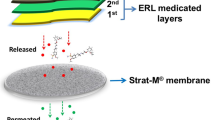
Abstract
In vitro release studies are commonly used to assess the product performance of topical dosage forms. In such studies, the mass transport of drugs through synthetic membranes into a receiving chamber filled with a release medium is measured. The release medium is also passed through filtration membranes prior to chromatographic analysis. There are no official guidelines directing membrane selection for in vitro release studies or for filtration. Considering the diversity in membrane materials and their physical properties, the aim of this study was to investigate membrane-drug binding and the effect of various membranes on the release performance of a model drug dexamethasone (DEX) using USP dissolution apparatus IV. Seven membranes of different pore sizes (0.45 and 1.2 μm) and materials (cellulose acetate, polyethersulfone, and nylon) were assessed. Two different methods, syringe filter and 24-h incubation, were used for the determination of membrane-drug binding effects at low drug concentrations and saturated concentration conditions. Cellulose acetate and nylon membranes showed significant drug binding after 24-h incubations at both drug concentrations. DEX diffusion through membranes was significantly slowed down in all the tested membranes when compared with DEX solution without membranes. The extent of the retardation varied due to the differences in membrane structures. In conclusion, materials and sources of membranes affected drug dissolution profiles and the results showed membrane-drug binding effects. Proper selection of membranes with low drug binding ability and low diffusion resistance is essential to ensure appropriate and reproducible in vitro release assessments and filtration studies.

Graphical Abstract







Similar content being viewed by others
References
Ueda C, Shah V, Derdzinski K, Ewing G, Flynn G, Maibach H, et al. Topical and transdermal drug products. Pharmacopeial Forum: The United States Pharmacopeial Convention, Inc.; 2009.
Bao Q, Shen J, Jog R, Zhang C, Newman B, Wang Y, et al. In vitro release testing method development for ophthalmic ointments. Int J Pharm. 2017;526:145–56.
Corbo M. Techniques for conducting in vitro release studies on semisolid formulations. Dissolution Technol. 1995;2:3–6.
Patere S, Newman B, Wang Y, Choi S, Mekjaruskul C, Jay M, et al. Influence of in vitro release methods on assessment of tobramycin ophthalmic ointments. Int J Pharm. 2020;590:119938.
Petró E, Paál TL, Eros I, Kenneth AS, Baki G, Csóka I. Drug release from semisolid dosage forms: a comparison of two testing methods. Pharm Dev Technol. 2015;20:330–6.
Azarmi S, Roa W, Löbenberg R. Current perspectives in dissolution testing of conventional and novel dosage forms. Int J Pharm. 2007;328:12–21.
Thakker KD, Chern WH. Development and validation of in vitro release tests for semisolid dosage forms—case study. Dissolution Technol. 2003:10–5.
Mafune E, Takahashi M, Takasugi N. In vivo and in vitro evaluations of water-absorption properties of various ointments. Drug Dev Ind Pharm. 1998;24:51–6.
Kanfer I, Rath S, Purazi P, Mudyahoto NA. In vitro release testing of semi-solid dosage forms. Dissolution Technol. 2017;24:52–60.
Olejnik A, Goscianska J, Nowak I. Active compounds release from semisolid dosage forms. J Pharm Sci. 2012;101:4032–45.
Ng SF, Rouse J, Sanderson D, Eccleston G. A comparative study of transmembrane diffusion and permeation of ibuprofen across synthetic membranes using franz diffusion cells. Pharmaceutics. 2010;2:209–23.
Wu ST, Shiu GK, Simmons JE, Bronaugh RL, Skelly JP. In vitro release of nitroglycerin from topical products by use of artificial membranes. J Pharm Sci. 1992;81:1153–6.
Gallagher SJ, Trottet L, Carter TP, Heard CM. Effects of membrane type and liquid/liquid phase boundary on in vitro release of ketoprofen form gel formulations. J Drug Target. 2003;11:373–9.
Nallagundla S, Patnala S, Kanfer I. Comparison of in vitro release rates of acyclovir from cream formulations using vertical diffusion cells. AAPS PharmSciTech. 2014;15:994–9.
Bao Q, Jog R, Shen J, Newman B, Wang Y, Choi S, et al. Physicochemical attributes and dissolution testing of ophthalmic ointments. Int J Pharm. 2017;523:310–9.
Dong Y, Qu H, Pavurala N, Wang J, Sekar V, Martinez MN, et al. Formulation characteristics and in vitro release testing of cyclosporine ophthalmic ointments. Int J Pharm. 2018;544:254–64.
Ng SF, Rouse JJ, Sanderson FD, Eccleston GM. The relevance of polymeric synthetic membranes in topical formulation assessment and drug diffusion study. Arch Pharm Res. 2012;35:579–93.
Marques MRC, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011;18:15–28.
Mohan Gandhi B, Lakshmana Rao A, Venkateswara RJ. Validated spectrophotometric and stability indicating RP-HPLC methods for the simultaneous estimation of gatifloxacin and dexamethasone in ophthalmic dosage form. Int J Chem Sci. 2016;14:617–34.
Sterlitech-Corporation. Advantages of asymmetric membranes [Internet]. 2018. Available from: https://www.sterlitech.com/blog/post/advantages-of-asymmetric-membranes
U.S. Department of Health and Human Services. FDA-SUPAC-SS. Guidance for industry. Non-sterile semisolid dosage forms scale-up and postapproval change: chemistry, manufacturing and controls. In vitro release testing and in vivo bioequivalence documentation U.S. 1997.
Kanke M, Eubanks JL, DeLuca PP. Binding of selected drugs to a “treated” inline filter. Am J Hosp Pharm. 1983;40:1323–8.
Funding
This study was supported by the U.S. Food and Drug Administration through Grant No. HHSF223201810114C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Disclaimer
The views expressed in this paper do not reflect the official policies of the U.S. Food and Drug Administration or the U.S. Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Additional information
Guest Editors: Qingguo Xu and Iok-Hou Pang
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mekjaruskul, C., Beringhs, A., Luo, WC. et al. Impact of Membranes on In Vitro Release Assessment: a Case Study Using Dexamethasone. AAPS PharmSciTech 22, 42 (2021). https://doi.org/10.1208/s12249-020-01874-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01874-y