Abstract
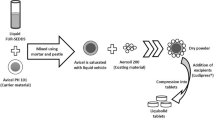
The aim of the present investigation was to formulate self-microemulsifying drug delivery system (SMEDDS) tablets to enhance the oral bioavailability of tizanidine hydrochloride. SMEDDS was prepared by using Capmul G as the oil phase, Tween 20 as the surfactant, and propylene glycol as the co-surfactant. The optimized formulation was characterized by dilution test, % transmittance, thermodynamic stability, dye solubility, assay, globule size, zeta potential, and TEM. A dye solubility test confirmed the formation of o/w microemulsion. Optimized formulation of SMEDDS had a drug content of 98 ± 0.75% (3.2± 0.3 mg) and droplet size of 96.61 ± 2.3 nm. Dilution and centrifugation tests indicated the physical stability of the formulation. The optimized SMEDDS was mixed with Neusilin as adsorbent, microcrystalline cellulose as diluent, and magnesium stearate as flow promoter, and compressed into tablets. The prepared tablets passed the tests of weight variation, hardness, friability, and assay. In vitro dissolution test indicated sustained release of tizanidine hydrochloride from the SMEDDS tablet for a period of 4 h. In vivo pharmacokinetic studies performed on male New Zealand rabbits showed a 4.61-fold increase in bioavailability compared with the marketed formulation. Thus, the developed SMEDDS tablet proved to be capable of enhancing oral bioavailability of tizanidine hydrochloride.

Graphical abstract





Similar content being viewed by others
References
[cited 2017 19th May]. Available from: http://www.aans.org/Patients/Neurosurgical-Conditions-and-Treatments/Spasticity.
Ipsen Announces FDA Approval of Dysport® (abobotulinumtoxinA) for the Treatment of Upper Limb Spasticity in children, Excluding Cerebral Palsy [cited 2020 4th April]. Available from: https://www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment.
Tizanidine Hydrochloride [cited 2020 26th May]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Tizanidine-hydrochloride.
Tse F, Jaffe J, Bhuta S. Pharmacokinetics of orally administered tizanidine in healthy volunteers. Fundamental & Clinical Pharmacology. 1987;1(6):479–88.
Granfors MT, Backman JT, Laitila J, Neuvonen PJ. Tizanidine is mainly metabolized by cytochrome p450 1A2 in vitro. Br J Clin Pharmacol. 2004;57(3):349–53.
Masareddy R, Kokate A, Shah V. Development of orodispersible tizanidine HCl tablets using spray dried coprocessed exipient bases. Indian J Pharm Sci. 2011;73(4):392–6.
Shanker G, Kumar CK, Gonugunta CSR, Kumar BV, Veerareddy PR. Formulation and evaluation of bioadhesive buccal drug delivery of tizanidine hydrochloride tablets. AAPS PharmSciTech. 2009;10(2):530–9.
Someshwar K, Chithaluru K, Ramarao T, Kumar K. Formulation and evaluation of effervescent floating tablets of tizanidine hydrochloride. Acta Pharma. 2011;61(2):217–26.
Hetal T, Bindesh P, Sneha T. A review on techniques for oral bioavailability enhancement of drugs. International Journal of Pharmaceutical Sciences Review and Research. 2010;4(3):033.
Kalepu S, Manthina M, Padavala V. Oral lipid-based drug delivery systems–an overview. Acta Pharm Sin B. 2013;3(6):361–72.
Shrestha H, Bala R, Arora S. Lipid-based drug delivery systems. Journal of pharmaceutics. 2014;2014:1–10.
Parmar N, Singla N, Amin S, Kohli K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Colloids Surf B: Biointerfaces. 2011;86(2):327–38.
Azeem A, Rizwan M, Ahmad FJ, Iqbal Z, Khar RK, Aqil M, et al. Nanoemulsion components screening and selection: a technical note. AAPS PharmSciTech. 2009;10(1):69–76.
Shakeel F, Haq N, El-Badry M, Alanazi FK, Alsarra IA. Ultra fine super self-nanoemulsifying drug delivery system (SNEDDS) enhanced solubility and dissolution of indomethacin. J Mol Liq. 2013;180:89–94.
Kamboj S, Rana V. Quality-by-design based development of a self-microemulsifying drug delivery system to reduce the effect of food on nelfinavir mesylate. Int J Pharm. 2016;501(1–2):311–25.
Vohra AM, Patel CV, Kumar P, Thakkar HP. Development of dual drug loaded solid self microemulsifying drug delivery system: exploring interfacial interactions using QbD coupled risk based approach. J Mol Liq. 2017;242:1156–68.
Food, Administration D. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Center for Drug Evaluation and Research (CDER). 2005:7.
Ali T, Shoaib MH, Yousuf RI, Siddiqui F, Ali H, Ahmed FR, et al. Development and validation of a reverse phase high performance liquid chromatography (HPLC) method for determination of tizanidine in human plasma. Afr J Pharm Pharmacol. 2014;8(7):199–205.
Guideline IHT. Stability testing of new drug substances and products. Q1A (R2), current step. 2003;4:1–24.
capmul-multi-functional-lipids [cited 2020 4th April]. Available from: https://www.abiteccorp.com/en/product-repository/capmul-multi-functional-lipids/.
Gurram A, Deshpande PB, Kar SS, Nayak UY, Udupa N, Reddy M. Role of components in the formation of self-microemulsifying drug delivery systems. Indian J Pharm Sci. 2015;77(3):249–57.
Capmul G, Tween 20, propylene glycol [cited 2020 26th May]. Available from: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm.
Neusilin [cited 2020 26th May]. Available from: http://www.fujichemical.co.jp/english/newsletter/newsletter_pharma_0805.html.
Desai J, Thakkar H. Effect of particle size on oral bioavailability of darunavir-loaded solid lipid nanoparticles. J Microencapsul. 2016;33(7):669–78.
Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (part 2). Trop J Pharm Res. 2013;12(2):265–73.
Khan AA, Mudassir J, Mohtar N, Darwis Y. Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int J Nanomedicine. 2013;8:2733.
Williams HD, Van Speybroeck M, Augustijns P, Porter CJ. Lipid-based formulations solidified via adsorption onto the mesoporous carrier Neusilin® US2: effect of drug type and formulation composition on in vitro pharmaceutical performance. J Pharm Sci. 2014;103(6):1734–46.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Approval of the experimental protocol was obtained from the Institutional Animal Ethics Committee of the Pharmacy department, The M. S. University of Baroda, Vadodara vide protocol no: MSU/IAEC/2016-17/1647. The experiments were conducted as per the requirements of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pramanik, S., Thakkar, H. Development of Solid Self-Microemulsifying System of Tizanidine Hydrochloride for Oral Bioavailability Enhancement: In Vitro and In Vivo Evaluation. AAPS PharmSciTech 21, 182 (2020). https://doi.org/10.1208/s12249-020-01734-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01734-9