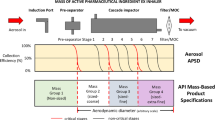
Abstract
Multi-stage cascade impactors (CI) are accepted for the determination of metrics of the drug mass aerodynamic particle size distributions (APSD) of aerosols emitted from orally inhaled products (OIPs). This is particularly important for products where the drug to excipient ratio or particle density may not be the same in each aerodynamic size fraction; examples of such products are carrier-containing dry powder inhalers (DPIs) and suspension pressurized metered-dose inhalers (pMDIs). CI measurements have been used as the “gold standard” for acceptance of alternative methods of APSD assessment, such as laser diffraction for nebulized solutions. Although these apparatus are labor-intensive, they are accepted in regulatory submissions and quality control assessments because the mass of active pharmaceutical ingredient(s) in the aerosol can be quantified by chemical assay and measured particle size is based on the aerodynamic diameter scale that is predictive of deposition in the respiratory tract. Two of the most important factors that modify the ideal operation of an impactor are “particle bounce,” that is often accompanied by re-entrainment in the air flow passing the stage of interest, and electrostatic charge acquired by the particles during the preparation and aerosolization of the formulation when the inhaler is actuated. This article reviews how both factors can lead to biased APSD measurements, focusing on measurements involving pMDIs and DPIs, where these sources of error are most likely to be encountered. Recommendations are provided for the mitigation of both factors to assist the practitioner of these measurements.






Similar content being viewed by others
References
EMEA. Guideline on the Pharmaceutical Quality of Inhalation and Nasal Products. London June 21, 2006.
United States Food and Drug Administration. Draft Guidance for Industry: Metered Dose Inhaler (MDI) and Dry Powder Inhaler (DPI) Products - Quality Considerations. Silver Spring, MD Revised April 2018.
Mitchell JP, Nagel MW. Cascade impactors for the size characterization of aerosols from medical inhalers: their uses and limitations. J Aerosol Med. 2003;16(4):341–77.
United States Pharmacopeial Convention. Aerosols, nasal sprays, metered-dose inhalers and dry powder inhalers. United States Pharmacopeia 42/National Formulary 37, Chapter 601. Rockville, MD 2018.
European Directorate for Quality in Medicines and Healthcare. Preparations for inhalation: aerodynamic assessment of fine particles. European Pharmacopeia 10.0, Chapter 2.9.18. Strasbourg, FR 2019.
Kwok PCL, Chan H-K. Electrostatics of pharmaceutical inhalation aerosols. J Pharm Pharmacol. 2009;61(12):1587-1599. Available from https://doi.org/10.1211/jpp.61.12.0002
Hindle M, Byron PR, Miller NC. Cascade impaction methods for dry powder inhalers using the high flowrate Marple-Miller impactor. Int J Pharm 1996;134(1):137–146. Available from https://doi.org/10.1016/0378-5173(95)04493-0.
Nasr MM, Ross DL, Miller NC. Effect of drug load and plate coating on the particle size distribution of a commercial albuterol metered dose inhaler (MDI) determined using the Andersen and Marple-Miller cascade impactors. Pharm Res. 1997;14(10):1437–43.
Vaughan NP. The Anderson Impactor: calibration, wall losses and numerical simulation. J Aerosol Sci. 1989;20:67–90.
Rissler J, Asking L, Dreyer JK. A methodology to study Impactor particle Reentrainment and a proposed stage coating for the NGI. J Aerosol Med Pulm Drug Del 2009;22(4):309–316. Available from https://doi.org/10.1089/jamp.2008.0735.
Rao AK, Whitby KT. Non-ideal collection characteristics of inertial impactors—I. single-stage impactors and solid particles. J Aerosol Sci 1978;9(2):77–86. Available from https://doi.org/10.1016/0021-8502(78)90069-1.
Rao AK, Whitby KT. Non-ideal collection characteristics of inertial impactors—II. Cascade impactors. J Aerosol Sci 1978;9(2):87–100. Available from https://doi.org/10.1016/0021-8502(78)90070-8.
Dzubay TG, Hines LE, Stevens RK. Particle bounce errors in cascade impactors. Atmos Environ (1967). 1976;10(3):229–234. Available from https://doi.org/10.1016/0004-6981(76)90095-0.
Cheng Y-S, Yeh H-C. Particle bounce in cascade impactors. Environ Sci Technol. 1979;13(11):1392-6. Available from https://doi.org/10.1021/es60159a017
Marple VA, Olson BA, Miller NC. A low-loss cascade impactor with stage collection cups: calibration and pharmaceutical inhaler applications. Aerosol Sci Technol 1995;22(1):124-34. Available from https://doi.org/10.1080/02786829408959732
Olson BA, Marple VA, Mitchell JP, Nagel MW. Development and calibration of a low-flow version of the Marple-Miller Impactor (MMI™). Aerosol Sci Technol. 1998;29(4):307-14. Available from https://doi.org/10.1080/02786829808965571
Marple VA, Roberts DL, Romay FJ, Miller NC, Truman KG, Van Oort M, et al. Next generation pharmaceutical impactor (a new impactor for pharmaceutical inhaler testing). Part I: design. J Aerosol Med. 2003;16(3):283–99.
May KR. The cascade impactor: an instrument for sampling coarse aerosols. J Sci Instrum. 1945;22(10):187–95.
Mitchell J, Nagel M, Avvakoumova V, MacKay H, Ali R. The abbreviated Impactor measurement (AIM) concept: part 1—influence of particle bounce and re-entrainment—evaluation with a “dry” pressurized metered dose inhaler (pMDI)-based formulation. AAPS PharmSciTech. 2009;10(1):243-51. Available from https://doi.org/10.1208/s12249-009-9202-9
Mitchell J, Nagel M, Avvakoumova V, MacKay H, Ali R. The abbreviated impactor measurement (AIM) concept: part II—influence of evaporation of a volatile component—evaluation with a “droplet-producing” pressurized metered dose inhaler (pMDI)-based formulation containing ethanol as Cosolvent. AAPS PharmSciTech. 2009;10(1):252-7. Available from https://doi.org/10.1208/s12249-009-9202-9
Chavan V, Dalby R. Novel system to investigate the effects of inhaled volume and rates of rise in simulated inspiratory air flow on fine particle output from a dry powder inhaler. AAPS Pharmaceutical Science. 2002;4(2):6.
Wei X, Hindle M, Delvadia RR, Byron PR. In vitro tests for aerosol deposition. V: using realistic testing to estimate variations in aerosol properties at the trachea J Aerosol Med Pulm Drug Del. 2017;30(5):339-48. Available from https://doi.org/10.1089/jamp.2016.1349
Taki M, Marriott C, Zeng X-M, Martin GP. Aerodynamic deposition of combination dry powder inhaler formulations in vitro: a comparison of three impactors. Int J Pharm. 2010;388(1-2):40-51. Available from https://doi.org/10.1016/j.ijpharm.2009.12.031
Leung SSY, Tang P, Zhou Q, Tong Z, Leung C, Decharaksa J, et al. De-agglomeration effect of the US pharmacopeia and Alberta throats on carrier-based powders in commercial inhalation products. AAPS J. 2015;17(6):1407-16. Available from https://doi.org/10.1208/s12248-015-9802-0
Khalili SF, Ghanbarzadeh S, Nokhodchi A, Hamishehkar H. The effect of different coating materials on the prevention of powder bounce in the next generation impactor. Res Pharm Sci. 2018;13(3):283-7. Available from https://doi.org/10.4103/1735-5362.228958
Mitchell JP, Nagel MW, Doyle CC, Ali RS, Avvakoumova VI, Christopher JD, et al. Relative precision of inhaler aerodynamic particle size distribution (APSD) metrics by full resolution and abbreviated Andersen cascade impactors (ACIs): part 2—investigation of Bias in extra-fine mass fraction with AIM-HRT impactor. AAPS PharmSciTech. 2010;11(3):1115-8. Available from https://doi.org/10.1208/s12249-010-9473-1
Nichols SC, Mitchell JP, Sandell D, Andersson PU, Fischer M, Howald M, et al. A multi-laboratory in vitro study to compare data from abbreviated and Pharmacopeial Impactor measurements for orally inhaled products: a report of the European Aerosol Group (EPAG). AAPS PharmSciTech. 2016;17(6):1383-92. Available from https://doi.org/10.1208/s12249-015-0476-9
Despres-Gnis F, Williams G, Comparison of next generation impactor and fast-screening impactor for determining fine particle fraction of dry powder inhalers. In: Drug Delivery to the Lungs 21; 2010,
Yoshida H, Kuwana A, Shibata H, Izutsu K-i, Goda Y. Comparison of aerodynamic particle size distribution between a next generation impactor and a cascade impactor at a range of flow rates. AAPS PharmSciTech. 2017;18(3):646-53. Available from https://doi.org/10.1208/s12249-016-0544-9
Lai C-Y, Huang S-H, Chang C-P, Lin J-Y. Reducing particle bounce and loading effect for a multi-hole Impactor. Aerosol Sci Technol. 2008;42(2):114-22. Available from https://doi.org/10.1080/02786820701809045
Pak SS, Liu BYH, Rubow KL. Effect of coating thickness on particle bounce in inertial Impactors. Aerosol Sci Technol. 1992;16:141–50.
Miller NC, Ross DL, Nasr MM. Effect of formulation factors on the observed bounce in cascade impactors used to measure the spray particle size of metered dose inhalers. Int J Pharm 1998;173(1):93–102. Available from https://doi.org/10.1016/S0378-5173(98)00217-8.
Turner JR, Hering SV. Greased and oiled substrates as bounce-free impaction surfaces. J Aerosol Sci 1987;18(2):215–224. Available from https://doi.org/10.1016/0021-8502(87)90057-7.
Kamiya A, Sakagami M, Hindle M, Byron PR. Aerodynamic sizing of metered dose inhalers: an evaluation of the Andersen and next generation pharmaceutical Impactors and their USP methods. J Pharm Sci. 2004;93(7):1828–37.
Kamiya A, Sakagami M, Byron PR. Cascade impactor practice for a high dose dry powder inhaler at 90 L/min: NGI versus modified 6-stage and 8-stage ACI. J Pharm Sci 2009;98(3):1028–1039. Available from https://doi.org/10.1002/jps.21501.
Rebits LG, Bennett DJ, Bhagwat PA, Morin A, Sievers RE. Method for quantifying the sample collected by an Andersen cascade impactor using total organic carbon analysis. J Aerosol Sci 2007;38(12):1197–1206. Available from https://doi.org/10.1016/j.jaerosci.2007.09.005.
Russell-Graham D, Cooper A, Stobbs B, McAulay E, Bogard H, Heath V, et al., Further evaluation of the fast-screening impactor for determining fine particle fraction of dry powder inhalers. In: Drug Delivery to the Lungs 21; 2010 The Aerosol Society, Edinburgh, UK.
Pantelides P, Bogard H, Russell-Graham D, Cooper A, Pitcairn G, Investigation into the use of the fast screening impactor as an abbreviated impactor measurement (AIM) tool for dry powder inhalers. In: Respiratory Drug Delivery Europe -2011; Dalby R, Byron P, Peart J, Suman J, Young P, eds. 2011, Davis HealthCare, River Grove, IL.
Wong W, Crapper J, Chan H-K, Traini D, Young PM. Pharmacopeial methodologies for determining aerodynamic mass distributions of ultra-high dose inhaler medicines. J Pharm Biomed Anal 2010;51(4):853–857. Available from https://doi.org/10.1016/j.jpba.2009.10.011.
Mitchell JP, Nagel MW, Doyle CC, Ali RS, Avvakoumova VI, Christopher JD, et al. Relative precision of inhaler aerodynamic particle size distribution (APSD) metrics by full resolution and abbreviated Andersen cascade impactors (ACIs): part 1. AAPS PharmSciTech. 2010;11(2):843-51. Available from https://doi.org/10.1208/s12249-010-9452-6
Chambers F, Smurthwaite M, Comparative performance evaluation of the Westech fine particle dose (FPD) impactor. In: Respiratory Drug Delivery-2012; Dalby R, Byron P, Peart J, Suman J, Young P, eds. 2012, Davis HealthCare, River Grove, IL.
Buttini F, Brambilla G, Copelli D, Sisti V, Balducci AG, Bettini R, et al. Effect of flow rate on in vitro aerodynamic performance of NEXThaler(®) in comparison with Diskus(®) and Turbohaler(®) dry powder inhalers. J Aerosol Med Pulm Drug Del. 2016;29(2):167-78. Available from https://doi.org/10.1089/jamp.2015.1220
Dunbar C, Kataya A, Tiangbe T. Reducing bounce effects in the Andersen cascade impactor. Int J Pharm 2005;301(1):25–32. Available from https://doi.org/10.1016/j.ijpharm.2005.04.039.
Mitchell JP, Costa PA, Waters S. An assessment of an Anderson mark II Cascade Impactor. J Aerosol Sci 1988;19(2):213–221. Available from https://doi.org/10.1016/0021-8502(88)90224-8.
Nichols SC, Brown DR, Smurthwaite M. New concept for the variable flow rate Andersen cascade impactor and calibration data. Journal of Aerosol Medicine. 1998;11:S133–S8.
Graham SJ, Lawrence RC, Ormsby ED, Pike RK. Particle size distribution of single and multiple sprays of salbutamol metered-dose inhalers (MDIs). Pharm Res. 1995;12(9):1380-4. Available from https://doi.org/10.1023/a:1016294228280
Nasr MM, Allgire JF. Loading effect on particle size measurements by inertial sampling of albuterol metered dose inhalers. Pharm Res. 1995;12(11):1677–81.
Stein SW, Turpin BJ, Cai X, Huang P-F, McMurry PH. Measurements of relative humidity -dependent bounce and density for atmospheric particles using the DMA-Impactor technique. Atmos Environ. 1994;28(10):1739–46.
Vasiliou JG, Sorensen D, McMurry PH. Sampling at controlled relative humidity with a cascade impactor. Atmos Environ 1999;33(7):1049–1056. Available from https://doi.org/10.1016/S1352-2310(98)00323-9.
Crouter A, Briens L. The effect of moisture on the flowability of pharmaceutical excipients. AAPS PharmSciTech. 2014;15(1):65–74.
Watts A, Wang Y-B, Johnston K, Williams R, III. Respirable low-density microparticles formed in situ from aerosolized brittle matrices. Pharm Res. 2013;30(3):813-25. Available from https://doi.org/10.1007/s11095-012-0922-2
Shemirani FM, Hoe S, Lewis D, Church T, Vehring R, Dr–Ing, et al. in vitro investigation of the effect of ambient humidity on regional delivered dose with solution and suspension MDIs. J aerosol med Pulm drug Deliv. 2013;26(4):215-22. Available from https://doi.org/10.1089/jamp.2012.0991
Longest PW, Hindle M. Condensational growth of combination drug-excipient submicrometer particles for targeted high efficiency pulmonary delivery: comparison of CFD predictions with experimental results. Pharm Res. 2012;29(3):707-21. Available from https://doi.org/10.1007/s11095-011-0596-1
Mitchell JP, Roberts DL. Current approaches to APSD measurements of OIP's based on inertial impaction. In: Tougas TP, Mitchell JP, Lyapustina SA, editors. Good Cascade Impactor practices, AIM and EDA for orally inhaled products. New York, NY: Springer; 2013. p. 15–55.
Grinshpun SA, Willeke K, Ulevicius V, Juozaitis A, Terzieva S, Donnelly J, Stelma GN, Brenner KP Effect of impaction, bounce and reaerosolization on the collection efficiency of Impingers. Aerosol Sci Technol 1997;26(4):326–342. Available from https://doi.org/10.1080/02786829708965434.
European Medicines Agency (EMA). Guideline on the requirements for clinical documentation for orally inhaled products (OIP) including the requirements for demonstration of therapeutic equivalence between two inhaled products for use in the treatment of asthma and chronic obstructive pulmonary disease (COPD) in adults and for use in the treatment of asthma in children and adolescents. London, UK 2009.
Leach C, Enhanced drug delivery through reformulating MDIs with HFA propellants—drug deposition and its effect on preclinical and clinical programs. In: Respiratory Drug Delivery–V; Dalby R, Byron P, Farr S, eds. 1996, Interpharm press, Buffalo Grove, IL.
Rogueda P, Morrical B, Chew YD, Comparison of NGI and the fast screening impactor (FSI) for suitability for analytical drug development. In: Drug Delivery to the Lungs 21; 2010 The Aerosol Society, Edinburgh, UK.
Wee Wallace B, Tavernini S, Martin Andrew R, Amirav I, Majaesic C, Finlay Warren H. Dry powder inhaler delivery of tobramycin in in vitro models of tracheostomized children. J Aerosol Med Pulm Drug Del. 2017;30(1):64-70. Available from https://doi.org/10.1089/jamp.2016.1309, 2017.
Zhou Y, Sun J, Cheng Y-S. Comparison of deposition in the USP and physical mouth–throat models with solid and liquid particles. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2011;24:277-84. Available from https://doi.org/10.1089/jamp.2011.0882
Tang P, Kwok PCL, Tong Z, Yang R, Raper JA, Chan H-K. Does the United States Pharmacopeia throat introduce De-agglomeration of carrier-free powder from inhalers? Pharmaceutical research. 2012;29:1797-807. Available from. https://doi.org/10.1007/s11095-012-0703-y.
Byron PR. Compendial dry powder testing: USP perspectives. In: Respiratory Drug Delivery IV; eds. 1994.
Kaialy W, Larhrib H, Martin G, Nokhodchi A. The effect of engineered mannitol-lactose mixture on dry powder inhaler performance. Pharm Res. 2012;29(8):2139-56. Available from https://doi.org/10.1007/s11095-012-0743-3
Mullins BJ, Agranovski IE, Braddock RD. Particle bounce during filtration of particles on wet and dry filters. Aerosol Sci Technol. 2003;37(7):587-600. Available from https://doi.org/10.1080/02786820300923
Roberts DL. Theory of multi-nozzle Impactor stages and the interpretation of stage mensuration data. Aerosol Sci Technol. 2009;43:1119–29.
Roberts DL, Mitchell JP. The next generation impactor (NGI)—manufacturing control: part II. Collection cups and the critical jet-to-plate distance Inhalation Magazine. 2016;10(4).
Roberts DL, Shelton CM. A practical method for eliminating type I and type II errors when assessing the suitability for continued use of a cascade impactor. Inhalation Magazine. 2018;12(2):12,4–8.
García-Ruiz E, Romay FJ, García JA, Cambra JF, Alonso L, Legarreta JA. Effect of nozzle spacing in the formation of primary and secondary deposits in multi-nozzle inertial impactors part I: experimental study. J Aerosol Sci 2019;136:61–81. Available from https://doi.org/10.1016/j.jaerosci.2019.06.008.
Christopher D, Curry P, Doub B, Furnkranz K, Lavery M, Lin K, Lyapustina S., Mitchell J., Rogers B., Strickland H., Tougas T., Tsong Y., Wyka B., PQRI Particle Size Distribution Mass Balance Working Group Considerations for the development and practice of cascade impaction testing, including a mass balance failure investigation tree. J Aerosol Med 2003;16(3):235–247. Available from https://doi.org/10.1089/089426803769017604.
Bonam M, Christopher D, Cipolla D, Donovan B, Goodwin D, Holmes S, et al. Minimizing variability of cascade impaction measurements in inhalers and nebulizers. AAPS PharmSciTech. 2008;9(2):404-13. Available from https://doi.org/10.1208/s12249-008-9045-9, 2008.
Karner S, Urbanetz NA. The impact of electrostatic charge in pharmaceutical powders with specific focus on inhalation-powders. J Aerosol Sci 2011;42(6):428–445. Available from https://doi.org/10.1016/j.jaerosci.2011.02.010.
Dunbar CA, Hickey AJ, Holzner P. Dispersion and characterization of pharmaceutical dry powder aerosols. KONA Powder Part J. 1998;16:7–45.
Stein SW, Rasmussen J, Walls S, Schultz DW, Oakley C, Drake JB. The influence of electrostatic controls on MDI size distributions measurements. AAPS PharmSciTech. 2019;20(#170).
Barry PW, O'callaghan C. The effect of delay, multiple actuations and spacer static charge on the in-vitro delivery of budesonide from the Nebuhaler. Br J Clin Pharmacol. 1995;40(1):76–8.
Barry PW, O'Callaghan C. The output of budesonide from spacer devices assessed under simulated breathing conditions. J Allergy Clin Immunol. 1999;104(6).
Vinchurkar S, Longest PW, Peart J. CFD simulations of the Andersen cascade impactor: model development and effects of aerosol charge. J Aerosol Sci 2009;40(9):807–822. Available from https://doi.org/10.1016/j.jaerosci.2009.05.005.
Mitchell JP, Coppolo DP, Nagel MW. Electrostatics and inhaled medications: influence on delivery via pressurized metered-dose inhalers and add-on devices. Respir Care. 2007;52(3):283–300.
Ijsebaert J, Geerse K, Marijnissen J, Lammers J, Zanen P. Electro-hydrodynamic atomization of drug solutions for inhalation purposes. J Appl Physiol. 2001;91:2735–41.
Hendricks C. Charging macroscopic particles. In: Moore AD, editor. Electrostatics and its applications. New York: John Wiley & Sons; 1973. p. 57–85.
Hoe S, Traini D, Chan H-K, Young PM. The influence of flow rate on the aerosol deposition profile and electrostatic charge of single and combination metered dose inhalers. Pharm Res. 2009;26(12):2639. Available from https://doi.org/10.1007/s11095-009-9979-y
Kwok PCL, Glover W, Chan HK. Electrostatic charge characteristics of aerosols produced from metered dose inhalers. J Pharm Sci 2005;94(12):2789–2799. Available from https://doi.org/10.1002/jps.20395.
Peart J, Orban JC, McGlynn P, Redmon MP, Sargeant CM, Byron PR. MDI electrostatics: valve and formulation interactions that really make a difference. Resp Drug Delivery VIII. 2002;8:223–30.
Elajnaf A, Carter P, Rowley G. Electrostatic characterisation of inhaled powders: effect of contact surface and relative humidity. Eur J Pharm Sci. 2006;29(5):375–84.
Peart J, Kulphaisal P, Orban J. Relevance of electrostatics in respiratory drug delivery. Business Briefings: Pharmagenerics. 2003:84–7.
Chen Y, Young PM, Fletcher DF, Chan HK, Long E, Lewis D, et al. The influence of actuator materials and nozzle designs on electrostatic charge of pressurised metered dose inhaler (pMDI) formulations. Pharm Res. 2014;31(5):1325-37. Available from https://doi.org/10.1007/s11095-013-1253-7
Chen Y, Young PM, Fletcher DF, Chan HK, Long E, Lewis D, et al. The effect of actuator nozzle designs on the electrostatic charge generated in pressurised metered dose inhaler aerosols. Pharm Res. 2015;32(4):1237-48. Available from https://doi.org/10.1007/s11095-014-1529-6
Chen Y, Young PM, Fletcher DF, Chan HK, Long E, Lewis D, et al. The effect of active pharmaceutical ingredients on aerosol electrostatic charges from pressurized metered dose inhalers. Pharm Res. 2015;32(9):2928-36. Available from https://doi.org/10.1007/s11095-015-1674-6
Kwok PCL, Noakes T, Chan H-K. Effect of moisture on the electrostatic charge properties of metered dose inhaler aerosols. J Aerosol Sci 2008;39(3):211–226. Available from https://doi.org/10.1016/j.jaerosci.2007.11.004.
Glover W, Chan H-K. Electrostatic charge characterization of pharmaceutical aerosols using electrical low-pressure impaction (ELPI). J Aerosol Sci 2004;35(6):755–764. Available from https://doi.org/10.1016/j.jaerosci.2003.12.003.
Hoe S, Young P, Chan H-K, Traini D. Introduction of the electrical next generation Impactor (eNGI) and investigation of its capabilities for the study of pressurized metered dose inhalers. Pharm Res 2009;26(2):431–437. Available from https://doi.org/10.1007/s11095-008-9761-6.
Byron PR, Peart J, Staniforth JN. Aerosol Electrostatics I: Properties of fine powders before and after Aerosolization by dry powder inhalers. Pharm Res. 1997;14(6):698-705. Available from https://doi.org/10.1023/a:1012181818244
Wong J, Kwok PCL, Niemelä V, Heng D, Crapper J, Chan H-K. Bipolar electrostatic charge and mass distributions of powder aerosols – effects of inhaler design and inhaler material. J Aerosol Sci 2016;95:104–117. Available from https://doi.org/10.1016/j.jaerosci.2016.02.003.
Hoe S, Traini D, Chan H-K, Young P. The contribution of different formulation components on the aerosol charge in carrier-based dry powder inhaler systems. Pharm Res. 2010;27(7):1325–36.
Hickey AJ, Mansour HM, Telko MJ, Xu Z, Smyth HDC, Mulder T, McLean R, Langridge J, Papadopoulos D Physical characterization of component particles included in dry powder inhalers. II. Dynamic characteristics. J Pharm Sci 2007;96(5):1302–1319. Available from https://doi.org/10.1002/jps.20943.
Telko MJ, Hickey AJ. Aerodynamic and electrostatic properties of model dry powder aerosols: a comprehensive study of formulation factors. AAPS PharmSciTech. 2014;15(6):1378-97. Available from https://doi.org/10.1208/s12249-014-0144-5
Kwok PCL, Chan H-K. Effect of relative humidity on the electrostatic charge properties of dry powder inhaler aerosols. Pharm Res 2008;25(2):277–288. Available from https://doi.org/10.1007/s11095-007-9377-2.
O'Connor DK, Tougas TP. Controlling analytical variability: a cascade impactor case study. Am Pharm Rev. 2007;10(2).
Huynh B, Wei X, Byron P, Hindle M, Evaluating pharmaceutical aerosol deposition in electrostatically charged plastic mouth-throat models. In: RDD Europe; Dalby R, Peart J, Suman J, Young P, Traini D, editors. 2017,
Huynh B, Young P, Farkas D, Longest W, Hindle M, D T, validating a realistic physical airway model to investigate in vitro drug deposition, dissolution and epithelial transport of orally inhaled products. In: Respiratory Drug Delivery, Europe-2017; Dalby R, Peart J, Suman J, Young P, Traini D, eds. 2017, Davis HealthCare River Grove, IL.
Chen Y, Keegstra J, An overview of the state of the art on mitigating electrostatic interference during aerodynamic testing on behalf of the European Pharmaceutical Aerosol Group (EPAG). In: Drug Delivery to the Lung; 2015, Mary Ann Liebert Inc.
Dewsbury NJ, Kenyon CJ, Newman SP. The effect of handling techniques on electrostatic charge on spacer devices: a correlation with in vitro particle size analysis. Int J Pharm. 1996;137(2):261–4.
Leung SSY, Chiow ACM, Kwok PCL, Ukkonen A, Chan H-K. Effect of spacers on the bipolar electrostatic charge properties of metered dose inhaler aerosols—a case study with Tilade®. J Pharm Sci 2017;106(6):1553–1559. Available from https://doi.org/10.1016/j.xphs.2017.02.014.
Bisgaard H, Anhøj J, Wildhaber J. Spacer devices. In: Bisgaard H, O’Callaghan C, Smaldone G, editors. Drug delivery to the lung. New York, NY: Marcel Dekker; 2000. p. 389–420.
European Medicines Agency. Guideline on the quality requirements for drug-device combinations. London 29 May 2019.
Health Canada. Guidance for industry: pharmaceutical quality of inhalation and nasal products. Ottawa October 1, 2006.
United States Food and Drug Administration 2018. Draft guidance for industry: metered dose inhaler (MDI) and dry powder inhaler (DPI) products—quality considerations. Silver Spring, MD.
US Pharmacopeial Convention. Spacers and valved holding chambers used with inhalation aerosols—characterization tests. Chapter 1602. Rockville, MD, USA 2018.
Wildhaber JH, Devadason SG, Hayden MJ, James R, Dufty AP, Fox RA, et al. Electrostatic charge an a plastic spacer device influences the delivery of salbutamol. Eur Respir J. 1996;9(9):1943-6. Available from https://doi.org/10.1183/09031936.96.09091943
Pierart F, Wildhaber JH, Vrancken I, Devadason SG, Le Souef PN. Washing plastic spacers in household detergent reduces electrostatic charge and greatly improves delivery. Eur Resp J. 1999;13(3):673-8. Available from. https://doi.org/10.1183/09031936.99.13367399.
Acknowledgments
The authors appreciate the support and comments from the wider IPAC-RS Cascade Impaction Working Group and IPAC-RS Board of Directors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doub, W., Stein, S., Mitchell, J. et al. Addressing the Need for Controls on Particle Bounce and Re-entrainment in the Cascade Impactor and for the Mitigation of Electrostatic Charge for Aerodynamic Particle Size Assessment of Orally Inhaled Products: an Assessment by the International Consortium on Regulation and Science (IPAC-RS). AAPS PharmSciTech 21, 239 (2020). https://doi.org/10.1208/s12249-020-01720-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01720-1