Abstract
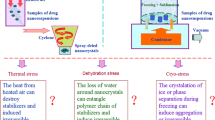
Commercial development of nanosuspensions for oral drug delivery generally involves a drying step which aims to generate a stable product that rapidly releases the nanocrystals once rehydrated and can be easily processed into a final dosage form (e.g., filled into hard capsule). Cryopelletisation is a freeze drying technique allowing the production of lyophilised micrometric spheres with good flowability. In the current work, the possibility to formulate redispersible ketoconazole nanocrystal-based cryopellets able to withstand intensive handling was investigated. Cryopellets were generated by first freezing regular droplets of nanosuspension using liquid nitrogen followed by water removal by sublimation in a standard freeze dryer. Low-friable cryopellets (< 1%) were produced by embedding the nanocrystals in a stabilizing hydroxypropyl cellulose SSL grade matrix, thus proving that these structures can withstand intensive handling. A threshold quantity of hydroxypropyl cellulose SSL grade (5/20 hydroxypropyl cellulose SSL grade-to-drug mass ratio) was required in combination with D-α-tocopherol polyethylene glycol 1000 succinate (vitamin E TPGS) to successfully recover the nanocrystals over storage. A further addition of micronised crospovidone has shown a positive effect on the dissolution performance of cryopellets. Altogether, this study demonstrated that the design of cryopellets combining the strengths of freeze-dried powders (porous internal structure, low residual humidity) and pellets (free-flowing units, mechanical resistance during handling) can potentially improve the nanocrystal’s redispersibility compared with other drying techniques while facilitating the downstream processing.






Similar content being viewed by others
References
Lipinski CA. Poor aqueous solubility - an industry wide problem in drug discovery. Am Pharm Rev. 2002;5:82–5.
Kesisoglou F, Panmai S, Wu Y. Nanosizing — oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59(7):631–44.
Gao L, Liu G, Ma J, Wang X, Zhou L, Li X. Drug nanocrystals: in vivo performances. J Control Release. 2012;160(3):418–30.
Peltonen L, Hirvonen J. Drug nanocrystals – versatile option for formulation of poorly soluble materials. Int J Pharm. 2018;537(1):73–83.
Junghanns J-UAH, Müller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomedicine. 2008;3(3):295–310.
Müller RH, Keck CM. Twenty years of drug nanocrystals: where are we, and where do we go? Eur J Pharm Biopharm. 2012;80(1):1–3.
Liversidge GG, Cundy KC. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int J Pharm. 1995;125(1):91–7.
Moschwitzer JP. Drug nanocrystals in the commercial pharmaceutical development process. Int J Pharm. 2013;453(1):142–56.
Peltonen L, Hirvonen J. Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol. 2010;62(11):1569–79.
Tuomela A, Hirvonen J, Peltonen L. Stabilizing agents for drug nanocrystals: effect on bioavailability. Pharmaceutics. 2016;8(2):16.
Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172(3):1126–41.
Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63(6):456–69.
Chin WW, Parmentier J, Widzinski M, Tan EH, Gokhale R. A brief literature and patent review of nanosuspensions to a final drug product. J Pharm Sci. 2014;103(10):2980–99.
Bhakay A, Azad M, Bilgili E, Dave R. Redispersible fast dissolving nanocomposite microparticles of poorly water-soluble drugs. Int J Pharm. 2014;461(1–2):367–79.
Cerdeira AM, Mazzotti M, Gander B. Formulation and drying of miconazole and itraconazole nanosuspensions. Int J Pharm. 2013;443(1–2):209–20.
Van Eerdenbrugh B, Froyen L, Van Humbeeck J, Martens JA, Augustijns P, Van den Mooter G. Drying of crystalline drug nanosuspensions—the importance of surface hydrophobicity on dissolution behavior upon redispersion. Eur J Pharm Sci. 2008;35(1–2):127–35.
Yue P-F, Li Y, Wan J, Yang M, Zhu W-F, Wang C-H. Study on formability of solid nanosuspensions during nanodispersion and solidification: I. novel role of stabilizer/drug property. Int J Pharm. 2013;454(1):269–77.
Bose S, Schenck D, Ghosh I, Hollywood A, Maulit E, Ruegger C. Application of spray granulation for conversion of a nanosuspension into a dry powder form. Eur J Pharm Sci. 2012;47(1):35–43.
Hou Y, Shao J, Fu Q, Li J, Sun J, He Z. Spray-dried nanocrystals for a highly hydrophobic drug: increased drug loading, enhanced redispersity, and improved oral bioavailability. Int J Pharm. 2017;516(1):372–9.
Azad M, Moreno J, Bilgili E, Dave R. Fast dissolution of poorly water soluble drugs from fluidized bed coated nanocomposites: impact of carrier size. Int J Pharm. 2016;513(1–2):319–31.
Niwa T, Danjo K. Design of self-dispersible dry nanosuspension through wet milling and spray freeze-drying for poorly water-soluble drugs. Eur J Pharm Sci. 2013;50(3–4):272–81.
Ali ME, Lamprecht A. Spray freeze drying for dry powder inhalation of nanoparticles. Eur J Pharm Biopharm. 2014;87(3):510–7.
Wanning S, Suverkrup R, Lamprecht A. Pharmaceutical spray freeze drying. Int J Pharm. 2015;488(1–2):136–53.
Erber M, Lee G. Cryopellets based on amorphous organic calcium salts: production, characterization and their usage in coagulation diagnostics. Powder Technol. 2015;280:10–7.
Erber M, Lee G. Development of cryopelletization and formulation measures to improve stability of Echis carinatus venum protein for use in diagnostic rotational thromboelastometry. Int J Pharm. 2015;495(2):692–700.
Erber M, Lee G. Production and characterization of rapidly dissolving cryopellets. J Pharm Sci. 2015;104(5):1668–76.
Peeters OM, Blaton NM, De Ranter CJ. Cis-1-Acetyl-4-(4-{[2-(2,4-dichlorophenyl)-2-(1H-1-imidazolylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazine: ketoconazole. A crystal structure with disorder. Acta Crystallogr Sect B. 1979;35(10):2461–4.
Viseras C, Salem II, Galan ICR, Galan AC, Galindo AL. The effect of recrystallization on the crystal growth, melting point and solubility of ketoconazole. Thermochim Acta. 1995;268:143–51.
Kasper JC, Friess W. The freezing step in lyophilization: physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur J Pharm Biopharm. 2011;78(2):248–63.
Beirowski J, Inghelbrecht S, Arien A, Gieseler H. Freeze drying of nanosuspensions, 2: the role of the critical formulation temperature on stability of drug nanosuspensions and its practical implication on process design. J Pharm Sci. 2011;100(10):4471–81.
Donoso MD, Haskell RJ, Schartman RR. Surfactant choice and the physical stability of nanosuspensions as a function of pH. Int J Pharm. 2012;439(1–2):1–7.
Saxena A, Shah D, Padmanabhan S, Gautam SS, Chowan GS, Mandlekar S, et al. Prediction of pH dependent absorption using in vitro, in silico, and in vivo rat models: early liability assessment during lead optimization. Eur J Pharm Sci. 2015;76:173–80.
Beirowski J, Inghelbrecht S, Arien A, Gieseler H. Freeze-drying of nanosuspensions, part 3: investigation of factors compromising storage stability of highly concentrated drug nanosuspensions. J Pharm Sci. 2012;101(1):354–62.
Allison SD, Molina MC, Anchordoquy TJ. Stabilization of lipid/DNA complexes during the freezing step of the lyophilization process: the particle isolation hypothesis. Biochim Biophys Acta BBA - Biomembr. 2000;1468(1):127–38.
Trasi NS, Byrn SR. Mechanically induced amorphization of drugs: a study of the thermal behavior of cryomilled compounds. AAPS PharmSciTech. 2012;13(3):772–84.
Van den Mooter G, Wuyts M, Blaton N, Busson R, Grobet P, Augustijns P, et al. Physical stabilisation of amorphous ketoconazole in solid dispersions with polyvinylpyrrolidone K25. Eur J Pharm Sci. 2001;12(3):261–9.
Touzet A, Pfefferlé F, van der Wel P, Lamprecht A, Pellequer Y. Active freeze drying for production of nanocrystal-based powder: a pilot study. Int J Pharm. 2018;536(1):222–30.
Ali ME, Lamprecht A. Spray freeze drying as an alternative technique for lyophilization of polymeric and lipid-based nanoparticles. Int J Pharm. 2017;516(1):170–7.
Khoshkava V, Kamal MR. Effect of drying conditions on cellulose nanocrystal (CNC) agglomerate porosity and dispersibility in polymer nanocomposites. Powder Technol. 2014;261:288–98.
Lee J, Cheng Y. Critical freezing rate in freeze drying nanocrystal dispersions. J Control Release. 2006;111(1–2):185–92.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Touzet, A., Pfefferlé, F., Lamprecht, A. et al. Formulation of Ketoconazole Nanocrystal-Based Cryopellets. AAPS PharmSciTech 21, 50 (2020). https://doi.org/10.1208/s12249-019-1570-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1570-1