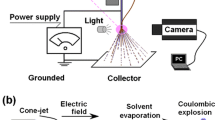
Abstract
Orodispersible films (ODFs) are more convenient for paediatric and geriatric patients to take as compared to conventional tablets and capsules. Electrospinning has recently been attempted to produce ODFs. This study investigated the feasibility of formulating poorly water-soluble drug into ODFs using electrospinning technology coupled with the anti-solvent precipitation method. Piroxicam (PX), a poorly water-soluble drug, was chosen as a model drug. Polyvinyl alcohol and polyvinylpyrrolidone were used as film forming polymers. PX microcrystals were prepared using poloxamer as the stabilizer with the anti-solvent precipitation method, and then loaded in ODFs through the electrospinning process. The obtained ODFs were characterized using a scanning electron microscope, X-ray powder diffraction and Fourier transform infrared spectroscopy with respect to the morphology, solid state and potential molecular interaction between the model drug and polymers. The mechanical property, disintegration and dissolution rate of the obtained ODF were evaluated using dynamic mechanical analysis, a customized method and USP2 apparatus. The results showed that PX microcrystals suspended in polymeric solutions could be readily electrospun into fibrous films, where the microcrystals scattered between the fibers. The electrospun fibrous film-based ODFs exhibited satisfactory mechanical behaviour, and fast disintegration upon the polymer selection. In the dissolution tests, almost 90% of PX was dissolved within 6 min from the ODFs, whereas 40% of PX dissolved from physical mixtures in 60 min. This study demonstrated that poorly water-soluble drugs could be formulated into ODFs with satisfactory quality attributes by combining micronization and the electrospinning process.








Similar content being viewed by others

References
Bhosle M, Benner JS, Dekoven M, Shelton J. Difficult to swallow: patient preferences for alternative valproate pharmaceutical formulations. Patient Prefer Adherence. 2009;3:161–71.
Borges AF, Silva C, Coelho JF, Simoes S. Oral films: current status and future perspectives: I—galenical development and quality attributes. J Control Release. 2015;206:1–19. https://doi.org/10.1016/j.jconrel.2015.03.006.
Hirani JJ, Rathod DA, Vadalia KR. Orally disintegrating tablets: a review. Trop J Pharm Res. 2009;8(2). https://doi.org/10.4314/tjpr.v8i2.44525.
Douroumis D. Orally disintegrating dosage forms and taste-masking technologies; 2010. Expert Opin Drug Deliv. 2011;8(5):665–75. https://doi.org/10.1517/17425247.2011.566553.
Venkatesh GM. Ondansetron orally disintegrating tablet compositions for prevention of nausea and vomiting. Google Patents. 2011.
Pilgaonkar P, Rustomjee M, Gandhi A, Bagde PM. Orally disintegrating tablets. Google Patents. 2013.
Elnaggar YS, El-Massik MA, Abdallah OY, Ebian AE. Maltodextrin: a novel excipient used in sugar-based orally disintegrating tablets and phase transition process. AAPS PharmSciTech. 2010;11(2):645–51. https://doi.org/10.1208/s12249-010-9423-y.
Arya A, Chandra A, Sharma V, Pathak K. Fast dissolving oral films: an innovative drug delivery system and dosage form. Int J Chem Tech Res. 2010;2(1):576–83.
Repka MA, Gutta K, Prodduturi S, Munjal M, Stodghill SP. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur J Pharm Biopharm. 2005;59(1):189–96. https://doi.org/10.1016/j.ejpb.2004.06.008.
Irfan M, Rabel S, Bukhtar Q, Qadir MI, Jabeen F, Khan A. Orally disintegrating films: a modern expansion in drug delivery system. Saudi Pharm J. 2016;24(5):537–46. https://doi.org/10.1016/j.jsps.2015.02.024.
Vuddanda PR, Mathew AP, Velaga S. Electrospun nanofiber mats for ultrafast release of ondansetron. React Funct Polym. 2016;99:65–72. https://doi.org/10.1016/j.reactfunctpolym.2015.12.009.
Krstic M, Radojevic M, Stojanovic D, Radojevic V, Uskokovic P, Ibric S. Formulation and characterization of nanofibers and films with carvedilol prepared by electrospinning and solution casting method. Eur J Pharm Sci. 2017;101:160–6. https://doi.org/10.1016/j.ejps.2017.02.006.
Yu DG, Yang JM, Branford-White C, Lu P, Zhang L, Zhu LM. Third generation solid dispersions of ferulic acid in electrospun composite nanofibers. Int J Pharm. 2010;400(1–2):158–64. https://doi.org/10.1016/j.ijpharm.2010.08.010.
Wang Q, Yu DG, Zhang LL, Liu XK, Deng YC, Zhao M. Electrospun hypromellose-based hydrophilic composites for rapid dissolution of poorly water-soluble drug. Carbohydr Polym. 2017;174:617–25. https://doi.org/10.1016/j.carbpol.2017.06.075.
Sakellariou P, Rowe R. Interactions in cellulose derivative films for oral drug delivery. Prog Polym Sci. 1995;20(5):889–942.
Mahesh A, Shastri N, Sadanandam M. Development of taste masked fast disintegrating films of levocetirizine dihydrochloride for oral use. Curr Drug Deliv. 2010;7(1):21–7.
Cilurzo F, Cupone IE, Minghetti P, Selmin F, Montanari L. Fast dissolving films made of maltodextrins. Eur J Pharm Biopharm. 2008;70(3):895–900. https://doi.org/10.1016/j.ejpb.2008.06.032.
Kunte S, Tandale P. Fast dissolving strips: a novel approach for the delivery of verapamil. J Pharm Bioallied Sci. 2010;2(4):325–8. https://doi.org/10.4103/0975-7406.72133.
El-Setouhy DA, Abd El-Malak NS. Formulation of a novel tianeptine sodium orodispersible film. AAPS PharmSciTech. 2010;11(3):1018–25. https://doi.org/10.1208/s12249-010-9464-2.
Tayel SA, El Nabarawi MA, Amin MM, Abou Ghaly MH. Sumatriptan succinate sublingual fast dissolving thin films: formulation and in vitro/in vivo evaluation. Pharm Dev Technol. 2016;21(3):328–37. https://doi.org/10.3109/10837450.2014.1003655.
Sagban TH, Ismail KY. Formulation and evaluation of orodispersible film of sildenafil citrate. Int J Pharm Pharm Sci. 2014;6(2):81–6.
Shimoda H, Taniguchi K, Nishimura M, Matsuura K, Tsukioka T, Yamashita H, et al. Preparation of a fast dissolving oral thin film containing dexamethasone: a possible application to antiemesis during cancer chemotherapy. Eur J Pharm Biopharm. 2009;73(3):361–5. https://doi.org/10.1016/j.ejpb.2009.08.010.
Kumar GP, Phani AR, Prasad RG, Sanganal JS, Manali N, Gupta R, et al. Polyvinylpyrrolidone oral films of enrofloxacin: film characterization and drug release. Int J Pharm. 2014;471(1–2):146–52. https://doi.org/10.1016/j.ijpharm.2014.05.033.
Dinge A, Nagarsenker M. Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity. AAPS PharmSciTech. 2008;9(2):349–56. https://doi.org/10.1208/s12249-008-9047-7.
Li X, Kanjwal MA, Lin L, Chronakis IS. Electrospun polyvinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin. Colloids Surf B: Biointerfaces. 2013;103:182–8. https://doi.org/10.1016/j.colsurfb.2012.10.016.
Illangakoon UE, Gill H, Shearman GC, Parhizkar M, Mahalingam S, Chatterton NP, et al. Fast dissolving paracetamol/caffeine nanofibers prepared by electrospinning. Int J Pharm. 2014;477(1–2):369–79. https://doi.org/10.1016/j.ijpharm.2014.10.036.
Tomar A, Sharma K, Chauhan NS, Mittal A, Bajaj U. Formulation and evaluation of fast dissolving oral film of dicyclomine as potential route of buccal delivery. Int J Drug Dev Res. 2012;4(2):408–17.
Susarla R, Sievens-Figueroa L, Bhakay A, Shen Y, Jerez-Rozo JI, Engen W, et al. Fast drying of biocompatible polymer films loaded with poorly water-soluble drug nano-particles via low temperature forced convection. Int J Pharm. 2013;455(1–2):93–103. https://doi.org/10.1016/j.ijpharm.2013.07.051.
Shen BD, Shen CY, Yuan XD, Bai JX, Lv QY, Xu H, et al. Development and characterization of an orodispersible film containing drug nanoparticles. Eur J Pharm Biopharm. 2013;85(3 Pt B):1348–56. https://doi.org/10.1016/j.ejpb.2013.09.019.
Sievens-Figueroa L, Bhakay A, Jerez-Rozo JI, Pandya N, Romanach RJ, Michniak-Kohn B, et al. Preparation and characterization of hydroxypropyl methyl cellulose films containing stable BCS class II drug nanoparticles for pharmaceutical applications. Int J Pharm. 2012;423(2):496–508. https://doi.org/10.1016/j.ijpharm.2011.12.001.
Hinton C, Pratt C, De Vadetzsky E, Landwill K, McCloskey K, Schuemann H. Twenty years of confectionery and chocolate progress. In: Twenty years of confectionery chocolate progress, vol. 111; 1970.
Won DH, Kim MS, Lee S, Park JS, Hwang SJ. Improved physicochemical characteristics of felodipine solid dispersion particles by supercritical anti-solvent precipitation process. Int J Pharm. 2005;301(1–2):199–208. https://doi.org/10.1016/j.ijpharm.2005.05.017.
Zimmermann A, Millqvist-Fureby A, Elema MR, Hansen T, Mullertz A, Hovgaard L. Adsorption of pharmaceutical excipients onto microcrystals of siramesine hydrochloride: effects on physicochemical properties. Eur J Pharm Biopharm. 2009;71(1):109–16. https://doi.org/10.1016/j.ejpb.2008.06.014.
Cho E, Cho W, Cha KH, Park J, Kim MS, Kim JS, et al. Enhanced dissolution of megestrol acetate microcrystals prepared by antisolvent precipitation process using hydrophilic additives. Int J Pharm. 2010;396(1–2):91–8. https://doi.org/10.1016/j.ijpharm.2010.06.016.
Zong X, Kim K, Fang D, Ran S, Hsiao BS, Chu B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer. 2002;43(16):4403–12.
Liu X, Nielsen LH, Klodzinska SN, Nielsen HM, Qu H, Christensen LP, et al. Ciprofloxacin-loaded sodium alginate/poly (lactic-co-glycolic acid) electrospun fibrous mats for wound healing. Eur J Pharm Biopharm. 2018;123:42–9. https://doi.org/10.1016/j.ejpb.2017.11.004.
Liu X, Aho J, Baldursdottir S, Bohr A, Qu H, Christensen LP, et al. The effect of poly (lactic-co-glycolic) acid composition on the mechanical properties of electrospun fibrous mats. Int J Pharm. 2017;529(1–2):371–80. https://doi.org/10.1016/j.ijpharm.2017.06.086.
Upadhyay PP, Bond AD. Crystallization and disorder of the polytypic α1 and α2 polymorphs of piroxicam. CrystEngComm. 2015;17(28):5266–72.
Lai F, Pini E, Corrias F, Perricci J, Manconi M, Fadda AM, et al. Formulation strategy and evaluation of nanocrystal piroxicam orally disintegrating tablets manufacturing by freeze-drying. Int J Pharm. 2014;467(1–2):27–33. https://doi.org/10.1016/j.ijpharm.2014.03.047.
Borodko Y, Habas SE, Koebel M, Yang P, Frei H, Somorjai GA. Probing the interaction of poly(vinylpyrrolidone) with platinum nanocrystals by UV-Raman and FTIR. J Phys Chem B. 2006;110(46):23052–9. https://doi.org/10.1021/jp063338+.
Zhang WX, Wang YZ, Sun CF. Characterization on oxidative stabilization of polyacrylonitrile nanofibers prepared by electrospinning. J Polym Res. 2007;14(6):467–74. https://doi.org/10.1007/s10965-007-9130-x.
Oksanen CA, Zografi G. Molecular mobility in mixtures of absorbed water and solid poly(vinylpyrrolidone). Pharm Res. 1993;10(6):791–9.
Koland M, Sandeep V, Charyulu N. Fast dissolving sublingual films of ondansetron hydrochloride: effect of additives on in vitro drug release and mucosal permeation. J Young Pharm: JYP. 2010;2(3):216–22.
Samprasit W, Akkaramongkolporn P, Ngawhirunpat T, Rojanarata T, Kaomongkolgit R, Opanasopit P. Fast releasing oral electrospun PVP/CD nanofiber mats of taste-masked meloxicam. Int J Pharm. 2015;487(1–2):213–22. https://doi.org/10.1016/j.ijpharm.2015.04.044.
Noyes AA, Whitney WR. The rate of solution of solid substances in their own solutions. J Am Chem Soc. 1897;19(12):930–4.
Vrecer F, Vrbinc M, Meden A. Characterization of piroxicam crystal modifications. Int J Pharm. 2003;256(1–2):3–15.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 73 kb)
Rights and permissions
About this article
Cite this article
Song, Q., Guo, X., Sun, Y. et al. Anti-solvent Precipitation Method Coupled Electrospinning Process to Produce Poorly Water-Soluble Drug-Loaded Orodispersible Films. AAPS PharmSciTech 20, 273 (2019). https://doi.org/10.1208/s12249-019-1464-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1464-2