Abstract
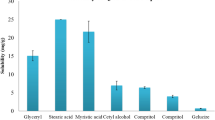
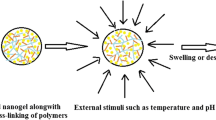
This work is devoted to design a novel nanostructured hybrid vesicle (NHV) made of lecithin and an acrylate/C10-C30 alkyl acrylate for the nasal delivery of a model active indomethacin (IND), and further to probe its microstructure, intermolecular interactions, drug release behavior, ex vivo permeation, and stability. NHVs were prepared by cavitation technology employing RSM-based central composite design (CCD). Amount of lecithin (X1), power of ultrasound (X2), and sonication time (X3) were selected as three independent variables while the studied response included Z-Avg (nm), polydispersity index (PDI), and zeta potential (mV). The designed system (NHV) was investigated through dynamic (DLS) and electrophoretic light scattering (ELS), attenuated total reflectance (ATR-FTIR), oscillatory measurement (stress and frequency sweep), and transmission electron microscopy (TEM). CCD was found useful in optimizing NHV. An optimized formulation (S6) had Z-Avg 80 nm, PDI 0.2, and zeta potential of − 43.26 mV. Morphology investigation revealed spherical vesicles with smaller TEM diameters (the largest particle being 52.26 nm). ATR analysis demonstrated significant intermolecular interactions among the drug (IND) and the components of vesicles. The designed vesicles had an elastic predominance and displayed supercase II (n > 1) type of drug release. Besides, the vesicles possessed potential to transport IND across the nasal mucosa with the steady-state flux (μg/cm2/h) and permeability coefficient (cm/h) of 26.61 and 13.30 × 10−3, respectively. NHV exhibited an exceptional stability involving a combination of electrostatic and steric interactions while the histopathology investigation confirmed their safety for nasal administration.








Similar content being viewed by others
References
Dao TPT, Brûlet A, Fernandes F, Er-Rafik M, Ferji K, Schweins R, et al. Mixing block copolymers with phospholipids at the nanoscale: from hybrid polymer/lipid worm-like micelles to vesicles presenting lipid nanodomains. Langmuir. 2017;33:1705–15.
Bixner O, Bello G, Virk M, Kurzhals S, Scheberl A, Gal N, et al. Magneto-thermal release from nanoscale unilamellar hybrid vesicles. ChemNanoMat. 2016;2:1111–20.
Chemin M, Brun PM, Lecommandoux S, Sandre O, Le Meins JF. Hybrid polymer/lipid vesicles: fine control of the lipid and polymer distribution in the binary membrane. Soft Matter. 2012;8:2867–74.
Cheng Z, Elias DR, Kamat NP, Johnston ED, Poloukhtine A, Popik V, et al. Improved tumor targeting of polymer-based nanovesicles using polymer-lipid blends. Bioconjug Chem. 2011;22:2021–9.
Dao TPT, Fernandes F, Er-Rafik M, Salva R, Schmutz M, Brulet A, et al. Phase separation and nanodomain formation in hybrid polymer/ lipid vesicles. ACS Macro Lett. 2015;4:182–6.
Henderson IM, Paxton WF. Salt, shake, fuse-giant hybrid polymer/lipid vesicles through mechanically activated fusion. Angew Chem Int Ed. 2014;53:3372–6.
Lim SK, de Hoog HP, Parikh AN, Nallani M, Liedberg B. Hybrid, nanoscale phospholipid/block copolymer vesicles. Polymers. 2013;5:1102–14.
Morimoto N, Sasaki Y, Mitsunushi K, Korchagina E, Wazawa T, Qiu XP, et al. Temperature-responsive telechelic dipalmitoylglyceryl poly (N-isopropylacrylamide) vesicles: real-time morphology observation in aqueous suspension and in the presence of giant liposomes. Chem Commun. 2014;50:8350–2.
Nam J, Beales PA, Vanderlick TK. Giant phospholipid/block copolymer hybrid vesicles: mixing behavior and domain formation. Langmuir. 2011;27(1):1–6.
Olubummo A, Schulz M, Schöps R, Kressler J, Binder WH. Phase changes in mixed lipid/polymer membranes by multivalent nanoparticle recognition. Langmuir. 2014;30:259–67.
Pippa N, Kaditi E, Pispas S, Demetzos C. PEO-b-PCL–DPPC chimeric nanocarriers: self-assembly aspects in aqueous and biological media and drug incorporation. Soft Matter. 2013;9:4073–82.
Pippa N, Stellas D, Skandalis A, Pispas S, Demetzos C, Libera M, et al. Chimeric lipid/block copolymer nanovesicles: physico-chemical and biocompatibility evaluation. Eur J Pharm Biopharm. 2016;107:295–309.
Ruysschaert T, Sonnen AFP, Haefele T, Meier W, Winterhalter M, Fournier D. Hybrid nanocapsules: interactions of ABA block copolymers with liposomes. J Am Chem Soc. 2005;127:6242–7.
Schulz M, Glatte D, Meister A, Scholtysek P, Kerth A, Blume A, et al. Hybrid lipid/polymer giant unilamellar vesicles: effects of incorporated biocompatible PIB–PEO block copolymers on vesicle properties. Soft Matter. 2011;7:8100–10.
Shen W, Hua J, Hu X. Impact of amphiphilic triblock copolymers on stability and permeability of phospholipid/polymer hybrid vesicles. Chem Phys Lett. 2014;600:56–61.
Su X, Mohamed Moinuddeen SK, Mori L, Nallani M. Hybrid polymersomes: facile manipulation of vesicular surfaces for enhancing cellular interaction. J Mater Chem B. 2013;1:5751–5.
Winzen S, Bernhardt M, Schaeffel D, Koch A, Kappl M, Koynov K, et al. Submicron hybrid vesicles consisting of polymer–lipid and polymer–cholesterol blends. Soft Matter. 2013;9:5883–90.
Kumbhar DD, Pokharkar VB. Physicochemical investigations on an engineered lipid–polymer hybrid nanoparticle containing a model hydrophilic active, zidovudine. Colloids Surf A Physicochem Eng Asp. 2013a;436:714–25.
Pokharkar VB, Jolly MR, Kumbhar DD. Engineering of a hybrid polymer–lipid nanocarrier for the nasal delivery of tenofovir disoproxil fumarate: physicochemical, molecular, microstructural, and stability evaluation. Eur J Pharm Sci. 2015;71:99–111.
Le Meins JF, Schatz C, Lecommandoux S, Sandre O. Hybrid polymer/lipid vesicles: state of the art and future perspectives. Mater Today. 2013;16:397–402.
Schulz M, Binder WH. Mixed hybrid lipid/polymer vesicles as a novel membrane platform. Macromol Rapid Commun. 2015;36:2031–41.
Sivakumar M, Tang SY, Tan KW. Cavitation technology—a greener processing technique for the generation of pharmaceutical nanoemulsions. Ultrason Sonochem. 2014;21:2069–83.
Gonzalez-Mira E, Egea MA, Garcia ML, Souto EB. Design and ocular tolerance of flurbiprofen loaded ultrasound-engineered NLC. Colloids Surf B: Biointerfaces. 2010;81:412–21.
Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–77.
Pelletier JP, Martel-Pelletier J, Rannou F, Cooper C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45:S22–7.
Takeuch K. Prostaglandin EP receptors and their roles in mucosal protection and ulcer healing in the gastrointestinal tract. Adv Clin Chem. 2010;51:121–44.
Kalra J, Khan A. Reducing Aβ load and tau phosphorylation: emerging perspective for treating Alzheimer’s disease. Eur J Pharmacol. 2015;764:571–81.
Tomita T. Secretase inhibitors and modulators for Alzheimer’s disease treatment. Expert Rev Neurother. 2009;9:661–79.
Bhugra C, Shmeis R, Pikal MJ. Role of mechanical stress in crystallization and relaxation behavior of amorphous indomethacin. J Pharm Sci. 2008;97:4446–58.
Choi KO, Choe J, Suh S, Ko S. Positively charged nanostructured lipid carriers and their effect on the dissolution of poorly soluble drugs. Molecules. 2016;21:672–84.
Okumura T, Ishida M, Takayama K, Otsuka M. Polymorphic transformation of indomethacin under high pressures. J Pharm Sci. 2006;95:689–700.
Surwase SA, Boetker JP, Saville D, Boyd BJ, Gordon KC, Peltonen L, et al. Indomethacin: new polymorphs of an old drug. Mol Pharm. 2013;10:4472–80.
Aidarova S, Sharipova A, Krägel J, Miller R. Polyelectrolyte/surfactant mixtures in the bulk and at water/oil interfaces. Adv Colloid Interf Sci. 2014;205:87–93.
Behbahani ES, Ghaedi M, Abbaspour M, Rostamizadeh K. Optimization and characterization of ultrasound assisted preparation of curcumin-loaded solid lipid nanoparticles: application of central composite design, thermal analysis and X-ray diffraction techniques. Ultrason Sonochem. 2017;38:271–80.
Jiang HL, Yang JL, Shi YP. Optimization of ultrasonic cell grinder extraction of anthocyanins from blueberry using response surface methodology. Ultrason Sonochem. 2017;34:325–31.
Song CK, Balakrishnan P, Shim CK, Chung SJ, Chong S, Kim DD. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation. Colloids Surf B: Biointerfaces. 2012;92:299–304.
Dubey V, Mishra D, Dutta T, Nahar M, Saraf DK, Jain NK. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J Control Release. 2013;123:148–54.
Kumbhar DD, Pokharkar VB. Engineering of a nanostructured lipid carrier for the poorly water-soluble drug, bicalutamide: physicochemical investigations. Colloids Surf A Physicochem Eng Asp. 2013b;416:32–42.
Pund S, Rasve G, Borade G. Ex vivo permeation characteristics of venlafaxine through sheep nasal mucosa. Eur J Pharm Sci. 2013;48:195–201.
Samson G, Calera AG, Girod SD, Faure F, Decullier E, Paintaud G, et al. Ex vivo study of bevacizumab transport through porcine nasal mucosa. Eur J Pharm Biopharm. 2012;80:465–9.
Giannola LI, Caro VD, Giandalia G, Siragusa MG, Tripodo C, Florena AM, et al. Release of naltrexone on buccal mucosa: permeation studies, histological aspects and matrix system design. Eur J Pharm Biopharm. 2007;67:425–33.
Gupta BC, Guttman I. Statistics and probability with applications for engineers and scientists. Hoboken: Wiley; 2013.
Yolmeh M, Najafzadeh M. Optimization and modelling green bean's ultrasound blanching. Int J Food Sci Technol. 2014;49:2678–84.
O’Sullivan J, Murray B, Flynn C, Norton I. Comparison of batch and continuous ultrasonic emulsification processes. J Food Eng. 2015;167:114–21.
Kovalchuk NM, Starov VM. Aggregation in colloidal suspensions: effect of colloidal forces and hydrodynamic interactions. Adv Colloid Interf Sci. 2012;179–182:99–106.
Valenta C, Auner BG. The use of polymers for dermal and transdermal delivery. Eur J Pharm Biopharm. 2004;58:279–89.
Miastkowska MA, Banach M, Pulit-Prociak J, Sikora ES, Glogowska A, Zielina M. Statistical analysis of optimal ultrasound emulsification parameters in thistle oil nanoemulsions. J Surfactant Deterg. 2017;20:233–46.
Vincent B. Early (pre-DLVO) studies of particle aggregation. Adv Colloid Interf Sci. 2012;170:56–67.
Chang DP, Jankunec M, Barauskas J, Tiberg F, Nylander T. Adsorption of lipid liquid crystalline nanoparticles on cationic, hydrophilic, and hydrophobic surfaces. ACS Appl Mater Interfaces. 2012;4:2643–51.
Karthik P, Anandharamakrishnan C. Enhancing omega-3 fatty acids nanoemulsion stability and in-vitro digestibility through emulsifiers. J Food Eng. 2016;187:92–105.
Bhattacharjee S. DLS and zeta potential—what they are and what they are not? J Control Release. 2016;235:337–51.
Greene AC, Zhu J, Pochan DJ, Jia X, Kiick KL. Poly (acrylic acid-b-styrene) amphiphilic multiblock copolymers as building blocks for the assembly of discrete nanoparticles. Macromolecules. 2011;44:1942–51.
Xuan J, Pelletier M, Xia H, Zhao Y. Ultrasound-induced disruption of amphiphilic block copolymer micelles. Macromol Chem Phys. 2011;212:498–506.
Sharma P, Denny WA, Garg S. Effect of wet milling process on the solid state of indomethacin and simvastatin. Int J Pharm. 2009;380:40–8.
Güler G, Gärtner RM, Ziegler C, Mäntele W. Lipid-protein interactions in the regulated betaine symporter BetP probed by infrared spectroscopy. J Biol Chem. 2016;291:4295–307.
Mashaan NS, Karim MR. Investigating the rheological properties of crumb rubber modified bitumen and its correlation with temperature susceptibility. Mater Res. 2013;16:116–27.
Shahin M, Hady SA, Hammad M, Mortada N. Optimized formulation for topical administration of clotrimazole using pemulen polymeric emulsifier. Drug Dev Ind Pharm. 2011;37:559–68.
Bonacucina G, Martelli S, Palmieri GF. Rheological, mucoadhesive and release properties of carbopol gels in hydrophilic cosolvents. Int J Pharm. 2004;282:115–30.
Torres LG, Iturbe R, Snowden MJ, Chowdhry BZ, Leharne SA. Preparation of o/w emulsion stabilized by solid particles and their characterization by oscillatory rheology. Colloids Surf A Physicochem Eng Asp. 2007;302:439–48.
Li J, Wang X, Zhang T, Wang C, Huang Z, Luo X, et al. A review on phospholipids and their main applications in drug delivery systems. Asian J Pharm Sci. 2015;10:81–98.
Szucs M, Sandri G, Bonferoni MC, Caramella CM, Vaghi P, Szabo-Revesz P, et al. Mucoadhesive behaviour of emulsions containing polymeric emulsifier. Eur J Pharm Sci. 2008;34(4–5):226–35.
Kosmidis K, Rinaki E, Argyrakis P, Macheras P. Analysis of case II drug transport with radial and axial release from cylinders. Int J Pharm. 2003;254:183–8.
Kalogeras IM, Neagu ER. Interplay of surface and confinement effects on the molecular relaxation dynamics of nanoconfined poly (methyl methacrylate) chains. Eur Phys J E. 2004;14:193–204.
Shaw CL, Dymock RB, Cowin A, Wormald PJ. Effect of packing on nasal mucosa of sheep. J Laryngol Otol. 2000;114:506–9.
Donovan MD, Huang Y. Large molecule and particulate uptake in the nasal cavity: the effect of size on nasal absorption. Adv Drug Deliv Rev. 1998;29:147–55.
Callens C, Remon JP. Evaluation of starch–maltodextrin–Carbopol 974 P mixtures for the nasal delivery of insulin in rabbits. J Control Release. 2000;66:215–20.
Hayashi K, Shimanouchi T, Kato K, Miyazaki T, Nakamura A, Umakoshi H. Span 80 vesicles have a more fluid, flexible and wet surface than phospholipid liposomes. Colloids Surf Biointerfaces. 2011;87:28–35.
Kato K, Walde P, Koine N, Ichikawa S, Ishikawa T, Nagahama R, et al. Temperature sensitive nonionic vesicles prepared from span 80 (sorbitan monoloeate). Langmuir. 2008;24:10762–70.
Jacobs C, Muller RH. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm Res. 2002;19:189–94.
Acknowledgements
Authors wish to acknowledge North Maharashtra University (NMU), Jalgaon, for providing Zetasizer facility, HR Patel College of Pharmacy Shirpur for NanoPlus facility, and Indian Institute of Technology (IIT), Mumbai, India, for TEM analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patil, S.S., Kumbhar, D.D., Manwar, J.V. et al. Ultrasound-Assisted Facile Synthesis of Nanostructured Hybrid Vesicle for the Nasal Delivery of Indomethacin: Response Surface Optimization, Microstructure, and Stability. AAPS PharmSciTech 20, 97 (2019). https://doi.org/10.1208/s12249-018-1247-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-018-1247-1