Abstract
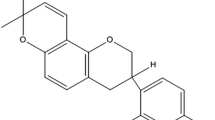
Proniosomes (PN) are the dry water-soluble carrier systems that may enhance the oral bioavailability, stability, and topical permeability of therapeutic agents. The low solubility and low oral bioavailability due to extensive first pass metabolism make Pentazocine as an ideal candidate for oral and topical sustained release delivery. The present study was aimed to formulate the PNs by quick slurry method that are converted to niosomes (liquid dispersion) by hydration, and subsequently formulated to semisolid niosomal gel. The PNs were found in spherical shape in the SEM and stable in the physicochemical and thermal analysis (FTIR, TGA, and XRD). The quick slurry method produced high recovery (> 80% yield) and better flow properties (θ = 28.1–37.4°). After hydration, the niosomes exhibited desirable entrapment efficiency (44.45–76.23%), size (4.98–21.3 μm), and zeta potential (− 9.81 to − 21.53 mV). The in vitro drug release (T100%) was extended to more than three half-lives (2–4 h) and showed good fit to Fickian diffusion indicated by Korsmeyer-Peppas model (n = 0.136–0.365 and R2 = 0.9747–0.9954). The permeation of niosomal gel was significantly enhanced across rabbit skin compared to the pure drug-derived gel. Therefore, the PNs are found promising candidates for oral as dissolution enhancement and sustained release for oral and topical delivery of pentazocine for the management of cancer pain.






Similar content being viewed by others
References
Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13(1):238-252. https://doi.org/10.1016/S0022-2836(65)80093-6.
Bangham A, Standish M, Weissmann G. The action of steroids and streptolysin S on the permeability of phospholipid structures to cations. J Mol Biol. 1965;13(1):253-259. https://doi.org/10.1016/S0022-2836(65)80094-8.
Deamer DW. From “Banghasomes” to liposomes: a memoir of Alec Bangham, 1921–2010. FASEB J. 2010;24(5):1308–10. https://doi.org/10.1096/fj.10-0503.
Toh M-R, Chiu GNC. Liposomes as sterile preparations and limitations of sterilisation techniques in liposomal manufacturing. Asian J Pharm Sci. 2013;8(2):88–95. https://doi.org/10.1016/j.ajps.2013.07.011.
Sharma A, Sharma US. Liposomes in drug delivery: progress and limitations. Int J Pharm. 1997;154(2):123–40. https://doi.org/10.1016/S0378-5173(97)00135-X.
Yoshida H, Lehr C-M, Kok W, Junginger H, Verhoef J, Bouwstra J. Niosomes for oral delivery of peptide drugs. J Control Release. 1992;21(1–3):145–53. https://doi.org/10.1016/0168-3659(92)90016-K.
Arzani G, Haeri A, Daeihamed M, Bakhtiari-Kaboutaraki H, Dadashzadeh S. Niosomal carriers enhance oral bioavailability of carvedilol: effects of bile salt-enriched vesicles and carrier surface charge. Int J Nanomedicine. 2015;10:4797-813.
Khan MI, Madni A, Ahmad S, Mahmood MA, Rehman M, Ashfaq M. Formulation design and characterization of a non-ionic surfactant based vesicular system for the sustained delivery of a new chondroprotective agent. Braz J Pharm Sci. 2015;51(3):607–15. https://doi.org/10.1590/S1984-82502015000300012.
Khan MI, Madni A, Peltonen L. Development and in-vitro characterization of sorbitan monolaurate and poloxamer 184 based niosomes for oral delivery of diacerein. Eur J Pharm Sci. 2016;95:88–95.
Madni A, Sarfraz M, Rehman M, Ahmad M, Akhtar N, Ahmad S, et al. Liposomal drug delivery: a versatile platform for challenging clinical applications. J Pharm Pharm Sci. 2014;17(3):401–26. https://doi.org/10.18433/J3CP55.
Eleje GU, Egeonu RO, Obianika C, Innocent I, JEO M, Osuagwu I, et al. Diclofenac and pentazocine versus pentazocine alone for post-operative analgesia in cesarean section. Int J Med Health Dev. 2015;20(2):381-400
Nersesyan H, Slavin KV. Current aproach to cancer pain management: availability and implications of different treatment options. Ther Clin Risk Manag. 2007;3(3):381.
Prasad Verma P, Chandak A. Development of matrix controlled transdermal delivery systems of pentazocine: in vitro/in vivo performance. Acta Pharma. 2009;59(2):171–86.
Paolino D, Cosco D, Muzzalupo R, Trapasso E, Picci N, Fresta M. Innovative bola-surfactant niosomes as topical delivery systems of 5-fluorouracil for the treatment of skin cancer. Int J Pharm. 2008;353(1):233–42. https://doi.org/10.1016/j.ijpharm.2007.11.037.
De Conno F, Ripamonti C, Sbanotto A, Barletta L, Zecca E, Martini C, et al. A clinical study on the use of codeine, oxycodone, dextropropoxyphene, buprenorphine, and pentazocine in cancer pain. J Pain Symptom Manag. 1991;6(7):423–7. https://doi.org/10.1016/0885-3924(91)90040-B.
Hu C, Rhodes DG. Proniosomes: a novel drug carrier preparation. Int J Pharm. 1999;185(1):23–35. https://doi.org/10.1016/S0378-5173(99)00122-2.
Blazek-Welsh AI, Rhodes DG. Maltodextrin-based proniosomes. Aaps Pharmsci. 2001;3(1):1–8. https://doi.org/10.1208/ps030101.
Rama K, Senapati P, Das M. Formulation and in vitro evaluation of ethyl cellulose microspheres containing zidovudine. J Microencapsul. 2005;22(8):863–76. https://doi.org/10.1080/02652040500273498.
Bansal S, Aggarwal G, Chandel P, Harikumar S. Design and development of cefdinir niosomes for oral delivery. J Pharm Bioallied Sci. 2013;5(4):318–25. https://doi.org/10.4103/0975-7406.120080.
Blazek-Welsh AI, Rhodes DG. SEM imaging predicts quality of niosomes from maltodextrin-based proniosomes. Pharm Res. 2001;18(5):656–61. https://doi.org/10.1023/A:1011037527889.
Wilkhu JS, Ouyang D, Kirchmeier MJ, Anderson DE, Perrie Y. Investigating the role of cholesterol in the formation of non-ionic surfactant based bilayer vesicles: thermal analysis and molecular dynamics. Int J Pharm. 2014;461(1):331–41. https://doi.org/10.1016/j.ijpharm.2013.11.063.
Ingham B. X-ray scattering characterisation of nanoparticles. Crystallogr Rev. 2015;21(4):229–303. https://doi.org/10.1080/0889311X.2015.1024114.
Gupta A, Prajapati SK, Singh M, Balamurugan M. Proniosomal powder of captopril: formulation and evaluation. Mol Pharm. 2007;4(4):596–9. https://doi.org/10.1021/mp0700110.
Abd-Elbary A, El-laithy HM, Tadros MI. Sucrose stearate-based proniosome-derived niosomes for the nebulisable delivery of cromolyn sodium. Int J Pharm. 2008;357(1–2):189–98. https://doi.org/10.1016/j.ijpharm.2008.01.056.
Abaee A, Madadlou A. Niosome-loaded cold-set whey protein hydrogels. Food Chem. 2016;196:106–13. https://doi.org/10.1016/j.foodchem.2015.09.037.
Solanki AB, Parikh JR, Parikh RH. Preparation, optimization and characterization of ketoprofen proniosomes for transdermal delivery. Int J Pharm Sci Nanotechnol. 2009;2:413–20.
Chakraborti CK, Sahoo S, Behera PK. Effect of different polymers on in vitro and ex vivo permeability of ofloxacin from its mucoadhesive suspensions. Saudi Pharm J. 2015;23(2):195–201. https://doi.org/10.1016/j.jsps.2014.08.003.
Sezgin-Bayindir Z, Yuksel N. Investigation of formulation variables and excipient interaction on the production of niosomes. AAPS PharmSciTech. 2012;13(3):826–35. https://doi.org/10.1208/s12249-012-9805-4.
Zhang J, Zhang X, Wang X, Huang Y, Yang B, Pan X, et al. The influence of maltodextrin on the physicochemical properties and stabilization of beta-carotene emulsions. AAPS PharmSciTech. 2016;17(1):1–8. https://doi.org/10.1208/s12249-016-0486-2.
Jamil B, Habib H, Abbasi S, Nasir H, Rahman A, Rehman A, et al. Cefazolin loaded chitosan nanoparticles to cure multi drug resistant Gram-negative pathogens. Carbohydr Polym. 2016;136:682–91. https://doi.org/10.1016/j.carbpol.2015.09.078.
Cascone MG, Lazzeri L, Carmignani C, Zhu Z. Gelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexate. J Mater Sci Mater Med. 2002;13(5):523–6.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. Am Assoc Pharm Sci. 2010;12(3):263–71.
Muzzalupo R. Niosomal drug delivery for transdermal targeting: recent advances. 2015;23-33.
Solanki A, Parikh J, Parikh R. Preparation, characterization, optimization, and stability studies of Aceclofenac Proniosomes. Iranian J Pharm Res. 2010;7(4):237–46.
Akhilesh D, Faishal G, Kamath J. Comparative study of carriers used in proniosomes. Int J Pharma Chem Sci. 2012;1:164–73.
Solanki AB, Parikh JR, Parikh RH. Formulation and optimization of piroxicam proniosomes by 3-factor, 3-level Box-Behnken design. AAPS PharmSciTech. 2007;8(4):43–9. https://doi.org/10.1208/pt0804086.
Gurrapu A, Jukanti R, Bobbala SR, Kanuganti S, Jeevana JB. Improved oral delivery of valsartan from maltodextrin based proniosome powders. Adv Powder Technol. 2012;23(5):583–90. https://doi.org/10.1016/j.apt.2011.06.005.
Koynov S, Muzzio FJ, Glasser BJ. A novel consolidation method to measure powder flow properties using a small amount of material. AICHE J. 2016;62(12):4193–200. https://doi.org/10.1002/aic.15321.
Wathoni N, Insani UC. Characterization and optimization of natural maltodextrin-based niosome. J Appl Pharm Sci. 2013;3(7):68-71.
Desai J, Alexander K, Riga A. Characterization of polymeric dispersions of dimenhydrinate in ethyl cellulose for controlled release. Int J Pharm. 2006;308(1):115–23. https://doi.org/10.1016/j.ijpharm.2005.10.034.
Sengodan T, Sunil B, Vaishali R, Chandra RJ, Nagar S, Nagar O. Formulation and evaluation of maltodextrin based proniosomes loaded with indomethacin. Int J PharmTech Res. 2009;1(3):517–23.
Ravaghi M, Sinico C, Razavi SH, Mousavi SM, Pini E, Fadda AM. Proniosomal powders of natural canthaxanthin: preparation and characterization. Food Chem. 2017;220:233–41. https://doi.org/10.1016/j.foodchem.2016.09.162.
Khalil RM, Abdelbary GA, Basha M, Awad GE, El-Hashemy HA. Design and evaluation of proniosomes as a carrier for ocular delivery of lomefloxacin HCl. J Liposome Res. 2016:1–12.
Sezgin-Bayindir Z, Antep MN, Yuksel N. Development and characterization of mixed niosomes for oral delivery using candesartan cilexetil as a model poorly water-soluble drug. AAPS PharmSciTech. 2015;16(1):108–17. https://doi.org/10.1208/s12249-014-0213-9.
Johnson SM. The effect of charge and cholesterol on the size and thickness of sonicated phospholipid vesicles. Biochim Biophys Acta Biomembr. 1973;307(1):27–41. https://doi.org/10.1016/0005-2736(73)90022-9.
Sahoo RK, Biswas N, Guha A, Sahoo N, Kuotsu K. Development and in vitro/in vivo evaluation of controlled release provesicles of a nateglinide–maltodextrin complex. Acta Pharm Sin B. 2014;4(5):408–16. https://doi.org/10.1016/j.apsb.2014.08.001.
Balakrishnan P, Shanmugam S, Lee WS, Lee WM, Kim JO, Oh DH, et al. Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int J Pharm. 2009;377(1-2):1–2):1-8. https://doi.org/10.1016/j.ijpharm.2009.04.020.
Akbari V, Abedi D, Pardakhty A, Sadeghi-Aliabadi H. Release studies on ciprofloxacin loaded non-ionic surfactant vesicles. Avicenna J Med Biotechnol. 2015;7(2):69.
El-Alim SA, Kassem A, Basha M. Proniosomes as a novel drug carrier system for buccal delivery of benzocaine. J Drug Deliv Sci Technol. 2014;24(5):452–8. https://doi.org/10.1016/S1773-2247(14)50087-1.
Shirsand S, Para M, Nagendrakumar D, Kanani K, Keerthy D. Formulation and evaluation of ketoconazole niosomal gel drug delivery system. Int J Pharm Investig. 2012;2(4):201–7. https://doi.org/10.4103/2230-973X.107002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madni, A., Rahim, M.A., Mahmood, M.A. et al. Enhancement of Dissolution and Skin Permeability of Pentazocine by Proniosomes and Niosomal Gel. AAPS PharmSciTech 19, 1544–1553 (2018). https://doi.org/10.1208/s12249-018-0967-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-0967-6