Abstract
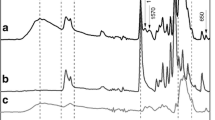
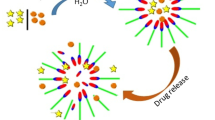
pH-sensitive N-naphthyl-N,O-succinyl chitosan (NSCS) and N-octyl-N,O-succinyl chitosan (OSCS) polymeric micelles carriers have been developed to incorporate curcumin (CUR) for colon-targeted drug delivery. The physical entrapment methods (dialysis, cosolvent evaporation, dropping, and O/W emulsion) were applied. The CUR-loaded micelles prepared by the dialysis method presented the highest loading capacity. Increasing initial amount of CUR from 5 to 40 wt% to polymer resulted in the increase in loading capacity of the polymeric micelles. Among the hydrophobic cores, there were no significant differences in the loading capacity of CUR-loaded micelles. The particle sizes of all CUR-loaded micelles were in the range of 120–338 nm. The morphology of the micelles changed after being contacted with medium with different pH values, confirming the pH-responsive properties of the micelles. The release characteristics of curcumin from all CUR-loaded micelles were pH-dependent. The percent cumulative release of curcumin from all CUR-loaded micelles in simulated gastric fluid (SGF) was limited to about 20%. However, the release amount was significantly increased after contacted with simulated intestinal fluid (SIF) (50–55%) and simulated colonic fluid (SCF) (60–70%). The released amount in SIF and SCF was significantly greater than the release of CUR from CUR powder. CUR-loaded NSCS exhibited the highest anti-cancer activity against HT-29 colorectal cancer cells. The stability studies indicated that all CUR-loaded micelles were stable for at least 90 days. Therefore, the colon targeted, pH-sensitive NSCS micelles may have potential to be a prospective candidate for curcumin delivery to the colon.









Similar content being viewed by others
References
Yokoyama M. Clinical application of polymeric micelle carrier systems in chemotherapy and image diagnosis of solid tumors. J Exp Clin Med. 2011;3(4):151–8.
Bromberg L. Polymeric micelles in oral chemotherapy. J Control Release. 2008;128(2):99–112.
Felber AE, Dufresne M-H, Leroux J-C. pH-sensitive vesicles polymeric micelles, and nanospheres prepared with polycarboxylates. Adv Drug Deliv Rev. 2012;64(11):979–92.
Xu W, Ling P, Zhang T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv. 2013;2013:15.
Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting-review article. Nanomed Nanotech Biol Med. 2010;6:714–29.
Zhang Y, Chen J, Zhang G, Lu J, Yan H, Liu K. Sustained release of ibuprofen from polymeric micelles with a high loading capacity of ibuprofen in media simulating gastrointestinal tract fluids. React Funct Polym. 2012;72(6):359–64.
Duan Y, Zhang B, Chu L, Tong HH, Liu W, Zhai G. Evaluation in vitro and in vivo of curcumin-loaded mPEG-PLA/TPGS mixed micelles for oral administration. Colloids Surf B: Biointerfaces. 2016;141:345–54.
Wang J, Ma W, Tu P. The mechanism of self-assembled mixed micelles in improving curcumin oral absorption: in vitro and in vivo. Colloids Surf B: Biointerfaces. 2015;133:198–19.
Mehanny M, Hathout RM, Geneidi AS, Mansour S. Exploring the use of nanocarrier systems to deliver the magical molecule; curcumin and its derivatives. J Control Release. 2016;225:1–30.
Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, et al. Chemopreventive effect of curcumin, a naturally occurring anti-inflamatory agent, during the promotion/progression stages of colon. Cancer Res. 1999;59(3):597–601.
Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55(2):259–66.
Shemesh N, Arber N. Curcumin alone and combination for prevention of colorectal cancer. Curr Colorectal Cancer Rep. 2014;10(1):62–7.
Cen L, Hutzen B, Ball S, DeAngelis S, Chen CL, Fuchs JR, et al. New structural analogues of curcumin exhibit potent growth suppressive activity in human colorectal carcinoma cells. BMC Cancer. 2009;9:99.
Guo L, Chen XJ, Hu YH, Yu ZJ, Wang D, Liu JZ. Curcumin inhibits proliferation and induces apoptosis of human colorectal cancer cells by activating the mitochondria apoptotic pathway. Phytother Res. 2013;27(3):422–30.
Akl MA, Kartal-Hodzic A, Oksanen T, Ismael HR, Afouna MM, Yliperttula M, et al. Factorial design formulation optimization and in vitro characterization of curcumin-loaded PLGA nanoparticles for colon delivery. J Drug Deliv Sci Technol. 2016;32:10–20.
Woraphatphadung T, Sajomsang W, Gonil P, Saesoo S, Opanasopit P. Synthesis and characterization of pH-responsive N-naphthyl-N,O-succinyl chitosan micelles for oral meloxicam delivery. Carbohydr Polym. 2015;121:99–106.
Woraphatphadung T, Sajomsang W, Gonil P, Treetong A, Akkaramongkolporn P, Ngawhirunpat T, et al. pH-Responsive polymeric micelles based on amphiphilic chitosan derivatives: effect of hydrophobic cores on oral meloxicam delivery. Int J Pharm. 2016;497:150–60.
Sajomsang W, Gonil P, Saesoo S, Ruktanonchai UR, Srinuanchai W, Puttipipatkhachorn S. Synthesis and anticervical cancer activity of novel pH responsive mocelles for oral curcumin delivery. Int J Pharm. 2014;477:261–72.
Opanasopit P, Yokoyama M, Watanabe M, Kawano K, Maitani Y, Okano T. Block copolymer design for camptothecin incorporation into polymeric micelles for passive tumor targeting. Pharm Res. 2004;21(10):2001–8.
Jiang GB, Quan D, Liao K, Wang H. Novel polymer micelles prepared from chitosan grafted hydrophobic palmitoyl groups for drug delivery. Mol Pharm. 2006;3(2):152–60.
Ngawhirunpat T, Wonglertnirant N, Opanasopit P, Ruktanonchai U, Yokson R, Wasanasuk K, et al. Incorporation methods for cholic acid chitosan-g-mPEG self-assembly micellar system containing camptothecin. Colloids Surf B: Biointerfaces. 2009;74(1):253–9.
Bu P, Narayanan S, Dalrymple D, Cheng X, Serajuddin AT. Cytotoxicity assessment of lipid-based self-emulsifying drug delivery system with Caco-2 cell model: cremophor EL as the surfactant. Eur J Pharm Sci. 2016;91:162–71.
Tu J, Xu Y, Xu J, Ling Y, Cai Y. Chitosan nanoparticles reduce LPS-induced inflammatory reaction via inhibition of NF-KB pathway in Caco-2 cells. Int J Biol Macromol. 2016;86:848–56.
Chae SY, Jang M, Nah J. Influence of molecular weight on oral absorption of water soluble chitosans. J Control Release. 2005;102(2):383–94.
Miller T, van Colen G, Sander B, Golas MM, Uezguen S, Weigandt M, et al. Drug loading of polymeric micelles. Pharm Res. 2013;30(2):584–95.
Yang YQ, Lin WJ, Zhao B, Wen XF, Guo XD, Zhang LJ. Synthesis and physicochemical characterization of amphiphilic triblock copolymer brush containing pH-sensitive linkage for oral drug delivery. Langmuir. 2012;28(21):8251–9.
Murthy RSR. Polymeric micelles in targeted drug delivery. In: Padma VD, Sanyog J, editors. Targeted drug delivery: concepts and design. USA: Springer; 2015. p. 501–40.
Qiu L, Li Z, Qiao M, Long M, Wang M, Zhang X, et al. Self-assembled pH-responsive hyaluronic acid-g-poly(Lhistidine) copolymer micelles for targeted intracellular delivery of doxorubicin. Acta Biomater. 2014;10(5):2024–35.
Zhang X, Liu B, Yang Z, Zhang C, Li H, Luo X, et al. Micelles of enzymatically synthesized PEG-poly(amine-co-ester) block copolymers as pH-responsive nanocarriers for docetaxel delivery. Colloids Surf B: Biointerfaces. 2014;115:349–58.
Yokoyama M, Kwon GS, Okano T, Sakurai Y, Kataoka K. Influencing factors on in vitro micelle stability of adriamycin-block copolymer conjugates. J Control Release. 1994;28:59–65.
Opanasopit O, Ngawhirunpat T, Rojanarata T, Choochottiros C, Chirachanchai S. Camptothecin-incorporating N-phthaloylchitosan-g-mPEG self-assembly micellar system: effect of degree of deacetylation. Colloids Surf B: Biointerfaces. 2007;60(1):117–24.
Li Y, Li H, Wei M, Lu J, Jin L. pH-Responsive composite based on prednisone-block copolymer micelle intercalated inorganic layered matrix: structure and in vitro drug release. Chem Eng J. 2009;151(1–3):359–66.
Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65(11):1631–52.
Lee W-H, Loo C-Y, Bebawy M, Luk F, Mason RS, Rohanizadeh R. Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol. 2013;11(4):338–78.
Rahman SMH, Telny TC, Ravi TK, Kuppusamy S. Role of surfactant and pH in dissolution of Curcumin. Indian J Pharm Sci. 2009;71(2):139–42.
Acknowledgements
This research was supported by the Commission of Higher Education (Thailand), the Thailand Research Fund through the Golden Jubilee Ph.D. Program (Grant No. PHD/0027/2556), the Thailand Research Fund, the National Nature Science Foundation of China (NSFC), and the Silpakorn University Research and Development Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Woraphatphadung, T., Sajomsang, W., Rojanarata, T. et al. Development of Chitosan-Based pH-Sensitive Polymeric Micelles Containing Curcumin for Colon-Targeted Drug Delivery. AAPS PharmSciTech 19, 991–1000 (2018). https://doi.org/10.1208/s12249-017-0906-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0906-y