Abstract
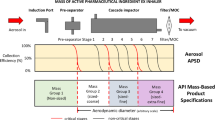
Wide variation in respiratory flow rates between patients emphasizes the importance of evaluating the aerodynamic particle size distribution (APSD) of dry powder inhaler (DPI) using a multi-stage impactor at different flow rates. US Pharmacopeia recently listed modified configurations of the Andersen cascade impactor (ACI) and new sets of cut-off diameter specifications for the operation at flow rates of 60 and 90 L/min. The purpose of this study was to clarify the effect of these changes on the APSD of DPI products at varied flow rates. We obtained APSD profiles of four DPIs and device combinations, Relenza®-Diskhaler® (GlaxoSmithKline Co.), Seebri®-Breezhaler® (Novartis Pharma Co.), Pulmicort®-Turbuhaler® (Astrazeneca Co.), and Spiriva®-Handihaler® (Nippon Boehringer Ingelheim Co.) using Next Generation Impactors (NGIs) and ACIs at flow rates from 28.3 to 90 L/min to evaluate the difference in the use of previous and new sets of cut-off diameter specifications. Processing the data using the new specifications for ACI apparently reduced large differences in APSD obtained by NGI and ACI with the previous specifications at low and high flow rates in all the DPIs. Selecting the appropriate configuration of ACI corresponding to the flow rate provided comparable APSD profiles of Pulmicort®-Turbuhaler® to those using NGIs at varied flow rates. The results confirmed the relevance of the current US Pharmacopeia specifications for ACI analysis in obtaining APSD profiles of DPI products at wide flow rates.








Similar content being viewed by others
References
Cheng YS. Mechanisms of pharmaceutical aerosol deposition in the respiratory tract. AAPS PharmSciTech. 2014;15:630–40.
Hoppentocht M, Hagedoorn P, Frijlink HW, de Boer AH. Technological and practical challenges of dry powder inhalers and formulations. Adv Drug Deliv Rev. 2014;75:18–31.
Newman SP. How well do in vitro particle size measurements predict drug delivery in vivo? J Aerosol Med. 1998;11 Suppl 1:S97–S104.
de Boer AH, Gjaltema D, Hagedoorn P. Inhalation characteristics and their effects on in vitro drug delivery from dry powder inhalers part 2: effect of peak flow rate (PIFR) and inspiration time on the in vitro drug release from three different types of commercial dry powder inhalers. Int J Pharm. 1996;138:45–56. doi:10.1016/0378-5173(96)04526-7.
Lee SL, Adams WP, Li BV, Conner DP, Chowdhury BA, Yu LX. In vitro considerations to support bioequivalence of locally acting drugs in dry powder inhalers for lung diseases. AAPS J. 2009;11:414–23.
Broeders ME, Molema J, Vermue NA, Folgering HT. In check dial: accuracy for Diskus and Turbuhaler. Int J Pharm. 2003;252:275–80.
EMA. Guideline on the requirements for clinical documentation for orally inhaled products (OIP) including the requirements for demonstration of therapeutic equivalence between two inhaled products for use in the treatment of asthma and chronic obstructive pulmonary disease (COPD) in adults and for use in the treatment of asthma in children and adolescents. Eur Med Agency; 2009.
US-FDA. Draft Guidance on Fluticasone Propionate; Salmeterol Xinafoate. U.S. Food and Drug Administration; 2013.
Marple VA, Roberts DL, Romay FJ, Miller NC, Truman KG, Van Oort M, et al. Next generation pharmaceutical impactor (a new impactor for pharmaceutical inhaler testing). Part I: design. J Aerosol Med. 2003;16:283–99.
Marple VA, Olson BA, Santhanakrishnan K, Mitchell JP, Murray SC, Hudson-Curtis BL. Next generation pharmaceutical impactor (a new impactor for pharmaceutical inhaler testing). Part II: archival calibration. J Aerosol Med. 2003;16:301–24.
Nichols SC. Calibration and mensuration issues for the standard and modified Andersen cascade impactor. Pharmeuropa. 2000;12:584–8.
Nichols SC, Brown DR, Smurthwaite M. New concept for the variable flow rate Andersen cascade impactor and calibration data. J Aerosol Med. 1998;11 Suppl 1:S133–8.
Kamiya A, Sakagami M, Byron PR. Cascade impactor practice for a high dose dry powder inhaler at 90 L/min: NGI versus modified 6-stage and 8-stage ACI. J Pharm Sci. 2009;98:1028–39.
Mitchell J, Nagel M. Particle size analysis of aerosols from medicinal inhalers. KONA. 2004;22:32–65.
Taki M, Marriott C, Zeng XM, Martin GP. Aerodynamic deposition of combination dry powder inhaler formulations in vitro: a comparison of three impactors. Int J Pharm. 2010;388:40–51.
Boonyapiwat B, Sarisuta N, Ma Y, Steventon GB. A validated HPLC method for zanamivir and its application to in vitro permeability study in Caco-2 culture model. Indian J Pharm Sci. 2011;73:564–8.
Trivedi RK, Chendake DS, Patel MC. A rapid, stability-indicating RP-HPLC method for the simultaneous determination of formoterol fumarate, tiotropium bromide, and ciclesonide in a pulmonary drug product. Sci Pharm. 2012;80:591–603.
Shur J, Lee S, Adams W, Lionberger R, Tibbatts J, Price R. Effect of device design on the in vitro performance and comparability for capsule-based dry powder inhalers. AAPS J. 2012;14:667–76.
Mitchell JP, Nagel MW. Cascade impactors for the size characterization of aerosols from medical inhalers: their uses and limitations. J Aerosol Med. 2003;16:341–77.
Mitchell JP, Costa PA, Waters S. An assessment of an Andersen mark-II cascade impactor. J Aerosol Sci. 1988;19:213–21. doi:10.1016/0021-8502(88)90224-8.
Vaughan NP. The Andersen impactor: calibration, wall losses and numerical simulation. J Aerosol Sci. 1989;20:67–90. doi:10.1016/0021-8502(89)90032-3.
Acknowledgments
This work was partly supported by the Research on Regulatory Harmonization and Evaluation of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics of the Japan Agency for Medical Research and Development, AMED, and by a Health and Labour Sciences Research Grant from the Ministry of Health, Labour, and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, H., Kuwana, A., Shibata, H. et al. Comparison of Aerodynamic Particle Size Distribution Between a Next Generation Impactor and a Cascade Impactor at a Range of Flow Rates. AAPS PharmSciTech 18, 646–653 (2017). https://doi.org/10.1208/s12249-016-0544-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0544-9