Abstract
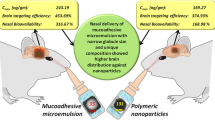
The objective of the present investigation was to optimize diazepam (Dzp)-loaded poly(lactic-co-glycolic acid) nanoparticles (NP) to achieve delivery in the brain through intranasal administration. Dzp nanoparticles (DNP) were formulated by nanoprecipitation and optimized using Box-Behnken design. The influence of various independent process variables (polymer, surfactant, aqueous to organic (w/o) phase ratio, and drug) on resulting properties of DNP (z-average and drug entrapment) was investigated. Developed DNP showed z-average 148–337 d.nm, polydispersity index 0.04–0.45, drug entrapment 69–92%, and zeta potential in the range of −15 to −29.24 mV. Optimized DNP were further analyzed by differential scanning calorimetry (DSC), Fourier transform infrared spectroscopy (FTIR), ex-vivo drug release, and in-vitro cytotoxicity. Ex-vivo drug release study via sheep nasal mucosa from DNP showed a controlled release of 64.4% for 24 h. 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay performed on Vero cell line showed less toxicity for DNP as compared to Dzp suspension (DS). Gamma scintigraphy and biodistribution study of DNP and DS was performed on Sprague-Dawley rats using technetium-99m-labeled (99mTc) Dzp formulations to investigate the nose-to-brain drug delivery pathway. Brain/blood uptake ratios, drug targeting efficiency, and direct nose-to-brain transport were found to be 1.23–1.45, 258, and 61% for 99mTc-DNP (i.n) compared to 99mTc-DS (i.n) (0.38–1.06, 125, and 1%). Scintigraphy images showed uptake of Dzp from nose-to-brain, and this observation was in agreement with the biodistribution results. These results suggest that the developed poly(D,L-lactide-co-glycolide) (PLGA) NP could serve as a potential carrier of Dzp for nose-to-brain delivery in outpatient management of status epilepticus.









Similar content being viewed by others
References
Shorvon S, Perucca E, Engel Jr J. The treatment of epilepsy. 3rd ed. UK: Wiley-Blackwell West Sussex; 2009.
Henney 3rd HR, Sperling MR, Rabinowicz AL, Bream G, Carrazana EJ. Assessment of pharmacokinetics and tolerability of intranasal diazepam relative to rectal gel in healthy adults. Epilepsy Res. 2014;108:1204–11.
Ivaturi VD, Riss JR, Kriel RL, Cloyd JC. Pharmacokinetics and tolerability of intranasal diazepam and midazolam in healthy adult volunteers. Acta Neurol Scand. 2009;120:353–7.
Sperling MR, Haas KF, Krauss G, Seif Eddeine H, Henney 3rd HR, Rabinowicz AL, et al. Dosing feasibility and tolerability of intranasal diazepam in adults with epilepsy. Epilepsia. 2014;55:1544–50.
Djupesland PG, Messina JC, Mahmoud RA. The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther Deliv. 2014;5:709–33.
Masserini M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013;2013:18. doi:10.1155/2013/238428.
Gizurarson S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr Drug Deliv. 2012;9:566–82.
Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–96.
Westin UE, Boström E, Gråsjö J, Hammarlund-Udenaes M, Björk E. Direct nose-to-brain transfer of morphine after nasal administration to rats. Pharm Res. 2006;23:565–72.
Shingaki T, Hidalgo IJ, Furubayashi T, Katsumi H, Sakane T, Yamamoto A, et al. The transnasal delivery of 5-fluorouracil to the rat brain is enhanced by acetazolamide (the inhibitor of the secretion of cerebrospinal fluid). Int J Pharm. 2009;377:85–91.
Patil SB, Sawant KK. Development, optimization and in vitro evaluation of alginate mucoadhesive microspheres of carvedilol for nasal delivery. J Microencapsul. 2009;26:432–43.
Reis CP, Neufeld RJ, Ribeiro ANJ, Veiga F. Nanoencapsulation I. methods for preparation of drug-loaded polymeric NP. Nanomedicine Nanotech Biol Med. 2006;2:8–21.
Danhier F, Ansorena E, Silva JM, Coco R, Breton AL, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–22.
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B. 2010;75:1–18.
Lu JM, Wang X, Muller CM, Wang H, Lin PH, Yao Q, et al. Current advances in research and clinical applications of PLGA based Nanotechnology. Expert Rev Mol Diagn. 2009;9:325–41.
Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–97.
Vergoni AV, Tosi G, Tacchi R, Vandelli MA, Bertolini A, Costantino L. Nanoparticles as drug delivery agents specific for CNS: in vivo biodistribution. Nanomedicine Nanotech Biol Med. 2009;5:369–77.
Feczko T, Toth J, Dosa G, Gyenis J. Influence of process conditions on the mean size of PLGA nanoparticles. Chem Eng Process. 2011;50:846–53.
Mainardes RM, Evangelista RC. PLGA nanoparticles containing praziquantel: effect of formulation variables on size distribution. Int J Pharm. 2005;290:7–144.
Sharma D, Maheshwari D, Philip G, Rana R, Bhatia S, Singh M, et al. Formulation and optimization of polymeric nanoparticles for intranasal delivery of lorazepam using Box-Behnken design: in vitro and in vivo evaluation. Biomed Res Int. 2014. doi:10.1155/2014/156010.
Budhian A, Siegel SJ, Winey KI. Haloperidol-loaded PLGA nanoparticles: systematic study of particle size and drug content. Int J Pharm. 2007;336:367–75.
Song X, Zhao Y, Hou S, Xu F, Zhao R, He J, et al. Dual agents loaded PLGA nanoparticles: systematic study of particle size and drug entrapment efficiency. Eur J Pharm Biopharm. 2008;69:445–53.
Songa KC, Lee HS, Chounga IY, Choa KI, Ahnb Y, Choi EJ. The effect of type of organic phase solvents on the particle size of poly(d, l-lactide-co-glycolide) nanoparticles. Colloids Surf A. 2006;276:162–7.
Hao J, Fang X, Wang J, Guo F, Li F, Peng X. Development and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a Box-Behnken design. Int J Nanomedicine. 2011;6:683–92.
Seju U, Kumar A, Sawant KK. Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: in vitro and in vivo studies. Acta Biomater. 2011;7:4169–76.
Fessi et al. Process for the preparation of dispersible colloidal systems of a substance in the form of nanoparticles. US Patent 1992; 5,118,528.
Bilati U, Emann EA, Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24:67–75.
Hornig S, Heinze T, Becer CR, Schubertb US. Nanoprecipitation and nanoformulation of polymers: from history to powerful possibilities beyond poly(lactic acid). Soft Matter. 2011;7:1581–8.
USP30-NF25, Diazepam, 1912.
Semete B, Booysen L, Lemmer Y, Kalombo L, Katata L, Jan V, et al. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine Nanotech Biol Med. 2010;6:662–71.
El-Ansary A, Al-Daihan S. On the toxicity of therapeutically used nanoparticles: an overview. J Toxicol 2009; 754810. doi:10.1155/2009/754810.
Lei R, Wu C, Yang B, Ma H, Shi C, Wang Q, et al. Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: a rapid in vivo screening method for nanotoxicity. Toxicol Appl Pharmacol. 2008;232:292–301.
Campos EVR, Melo NFSD, Guilherme VA, Paula ED, Rosa AH, ArauJo DRD, et al. Preparation and characterization of poly(ε-Caprolactone) nanospheres containing the local anesthetic lidocaine. J Pharm Sci. 2013;102:215–26.
Mathew A, Takahiro F, Yutaka N, Takashi H, Hisao M, Yasuhiko Y, et al. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE. 2012;12(7):e32616.
Snehalatha M, Kolachina V, Saha RN, Babbar AK, Sharma N, Sharma RK. Enhanced tumor uptake, biodistribution and pharmacokinetics of etoposide loaded nanoparticles in Dalton’s lymphoma tumor bearing mice. J Pharm Bioallied Sci. 2013;5:38–45.
Vyas TK, Babbar AK, Sharma RK, Misra A. Intranasal mucoadhesive microemulsions of zolmitriptan: preliminary studies on brain-targeting. J Drug Target. 2005;13:317–24.
Kumar M, Misra A, Babbar AK, Mishra AK, Mishra P, Pathak K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int J Pharm. 2008;358:285–91.
Kumar M, Misra A, Mishra AK, Mishra P, Pathak K. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J Drug Target. 2008;16:806–14.
Alam S, Khan ZI, Mustafa G, Kumar M, Islam F, Bhatnagar A, et al. Development and evaluation of thymoquinone- encapsulated chitosan nanoparticles for nose-to-brain targeting: a pharmacoscintigraphic study. Int J Nanomedicine. 2012;7:5705–18.
Panyam J, Williams D, Dash A, Leslie-Pelecky D, Labhasetwar V. Solid-state solubility influences encapsulation and release of hydrophobic drugs from PLGA/PLA nanoparticles. J Pharm Sci. 2004;93:1804–14.
Hanson LR, Frey WH. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9:1–4.
Averineni RK, Shavi GV, Gurram AK, Deshpande PB, Arumugam K, Maliyakkal N, et al. PLGA 50:50 nanoparticles of paclitaxel: development, in vitro anti-tumor activity in BT-549 cells and in vivo evaluation. Bull Mater Sci. 2012;35:319–26.
Nah JW, Y-Il J, Koh JJ. Drug release from nanoparticles of poly(dl-lactide-co-glycolide). Korean J Chem Eng. 2006;17:230–6.
Ivaturi V, Kriel R, Brundage R, Gordon L, Mansbach H, Cloyd J. Bioavailability of intranasal vs. rectal diazepam. Epilepsy Res. 2013;103:254–61.
Agarwal SK, Kriel RL, Brundage RC, Ivaturi VD, Cloyd JC. A pilot study assessing the bioavailability and pharmacokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res. 2013;105:362–7.
Betancourt T, Brown B, Brannon-Peppas L. Doxorubicin-loaded PLGA nanoparticles by nanoprecipitation: preparation, characterization and in vitro evaluation. Nanomedicine. 2007;2:219–32.
Sharma D, Gabrani R, Sharma SK, Ali J, Dang S. Development of midazolam loaded PLGA nanoparticles for treatment of status epilepticus. Adv Sci Lett. 2014;20:1526–30.
Prajapati RK, Mahajan HS, Surana SJ. PLGA based mucoadhesive microspheres for nasal delivery: in vitro/ex vivo studies. Indian J Novel Drug Deliv. 2011;3:9–16.
Quintanar-Guerrero D, Allemann E, Fessi H, Doelker E. Preparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymers. Drug Dev Ind Pharm. 1998;24:1113–28.
Sylaja B, Srinivasan S. Experimental and theoretical investigation of spectroscopic properties of diazepam. Int J Chem Tech Res. 2012;4:361–76.
Mainardes RM, Gremiao MPD, Evangelista RC. Thermoanalytical study of praziquantel-loaded PLGA nanoparticles. Braz J Pharm Sci. 2006;42:523–30.
van Woensel M, Wauthoz N, Rosière R, Amighi K, Mathieu V, Lefranc F, et al. Formulations for intranasal delivery of pharmacological agents to combat brain disease: a new opportunity to tackle GBM? Cancers. 2013;5:1020–48.
Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29:3867–76.
Sharma G, Mishra AK, Mishra P, Misra A. Intranasal cabergoline: pharmacokinetic and pharmacodynamic studies. AAPS PharmSciTech. 2009;10:1321–30.
Babu RJ, Dayal PP, Pawar K, Singh M. Nose-to-brain transport of melatonin from polymer gel suspensions: a microdialysis study in rats. J Drug Target. 2011;19:731–40.
Acknowledgments
The authors would like to thank the Department of Biotechnology, Government of India for providing financial support to conduct the research work (DBT project No. BT/PR1891/MED/30/626/2011). The authors are grateful to the Jaypee Institute of Information Technology, Noida, UP (India), for the infrastructural support, Director of INMAS and Dr. Aseem Bhatnagar, Nuclear Medicine Department, for providing nuclear scintigraphy facility, and Dr. A. K. Panda from the National Institute of Immunology, New Delhi (India), for providing the facility for particle size analysis and zeta potential.
Conflict of Interest
The authors declare that this paper content has no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, D., Sharma, R.K., Sharma, N. et al. Nose-To-Brain Delivery of PLGA-Diazepam Nanoparticles. AAPS PharmSciTech 16, 1108–1121 (2015). https://doi.org/10.1208/s12249-015-0294-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-015-0294-0