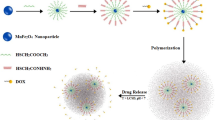
Abstract
Herein, we report the successful development of a novel nanosystem capable of an efficient delivery and temperature-triggered drug release specifically aimed at cancer. The water-soluble 130.1 ± 0.2 nm iron oxide nanoparticles (IONPs) were obtained via synthesis of a monodispersed iron oxide core stabilized with tetramethylammonium hydroxide pentahydrate (TMAOH), followed by coating with the thermoresponsive copolymer poly-(NIPAM-stat-AAm)-block-PEI (PNAP). The PNAP layer on the surface of the IONP undergoes reversible temperature-dependent structural changes from a swollen to a collapsed state resulting in the controlled release of anticancer drugs loaded in the delivery vehicle. We demonstrated that the phase transition temperature of the prepared copolymer can be precisely tuned to the desired value in the range of 36°C–44°C by changing the monomers ratio during the preparation of the nanoparticles. Evidence of modification of the IONPs with the thermoresponsive copolymer is proven by ATR-FTIR and a quantitative analysis of the polymeric and iron oxide content obtained by thermogravimetric analysis. When loaded with doxorubicin (DOX), the IONPs-PNAP revealed a triggered drug release at a temperature that is a few degrees higher than the phase transition temperature of a copolymer. Furthermore, an in vitro study demonstrated an efficient internalization of the nanoparticles into the cancer cells and showed that the drug-free IONPs-PNAP were nontoxic toward the cells. In contrast, sufficient therapeutic effect was observed for the DOX-loaded nanosystem as a function of temperature. Thus, the developed temperature-tunable IONPs-based delivery system showed high potential for remotely triggered drug delivery and the eradication of cancer cells.







Similar content being viewed by others
References
Lopes J, Santos G, Barata P, Oliveira R, Lopes CM. Physical and chemical stimuliresponsive drug delivery systems: targeted delivery and main routes of administration. Curr Pharm Des. 2013;Epub ahead of print.
Alexander C, Shakesheff KM. Responsive polymers at the biology/materials science interface. Adv Mater. 2006;18:3321–8.
Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22.
Cheng R, Meng FH, Deng C, Klok HA, Zhong ZY. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013;34:3647–57.
Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58:1655–70.
Muller-Schulte D, Schmitz-Rode T. Thermosensitive magnetic polymer particles as contactless controllable drug carriers. J Magn Magn Mater. 2006;302:267–71.
Eeckman F, Moes AJ, Amighi K. Synthesis and characterization of thermosensitive copolymers for oral controlled drug delivery. Eur Polymer J. 2004;40:873–81.
Laurent S, Dutz S, Hafeli UO, Mahmoudi M. Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv Colloid Interface Sci. 2011;166:8–23.
Liu XQ, Kaminski MD, Chen HT, Torno M, Taylor L, Rosengart AJ. Synthesis and characterization of highly-magnetic biodegradable poly(d, l-lactide-co-glycolide) nanospheres. J Control Release. 2007;119:52–8.
Laurent S, Mahmoudi M. Superparamagnetic iron oxide nanoparticles: promises for diagnosis and treatment of cancer. Int J Mol Epidemiol Genet. 2011;2:367–90.
Vihola H, Laukkanen A, Valtola L, Tenhu H, Hirvonen J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam). Biomaterials. 2005;26:3055–66.
Kuckling D, Adler HJP, Arndt KF, Ling L, Habicher WD. Temperature and pH dependent solubility of novel poly(N-isopropylacrylamide) copolymers. Macromol Chem Phys. 2000;201:273–80.
Principi T, Goh CCE, Liu RCW, Winnik FM. Solution properties of hydrophobically modified copolymers of N-isopropylacrylamide and N-glycine acrylamide: a study by microcalorimetry and fluorescence spectroscopy. Macromolecules. 2000;33:2958–66.
Hoogenboom R, Thijs HML, Jochems MJHC, Lankvelt BM, Fijten MWM, Schubert US. Tuning the LCST of poly(2-oxazoline)s by varying composition and molecular weight: alternatives to poly(N-isopropylacrylamide). Chem Comm. 2008;5758–60.
Zintchenko A, Ogris M, Wagner E. Temperature dependent gene expression induced by PNIPAM-based copolymers: potential of hyperthermia in gene transfer. Bioconjug Chem. 2006;17:766–72.
Narain R, Gonzales M, Hoffman AS, Stayton PS, Krishnan KM. Synthesis of monodisperse biotinylated p(NIPAAm)-coated iron oxide magnetic nanoparticles and their bioconjugation to streptavidin. Langmuir. 2007;23:6299–304.
Deng Y, Yang W-L, Wang C, Fu S. A novel approach for preparation of thermoresponsive polymer magnetic microspheres with core-structure. Adv Mater. 2003;15:1729–32.
Zhang S, Zhang L, He B, Wu Z. Preparation and characterization of thermosensitive PNIPAA-coated iron oxide nanoparticles. Nanothechnology. 2008;19:325608.
Zhang JL, Srivastava RS, Misra RDK. Core-shell magnetite nanoparticles surface encapsulated with smart stimuli-responsive polymer: synthesis, characterization, and LCST of viable drug-targeting delivery system. Langmuir. 2007;23:6342–51.
Liu G, Hu D, Chen M, Wang CC, Wu LM. Multifunctional PNIPAM/Fe3O4-ZnS hybrid hollow spheres: synthesis, characterization, and properties. J Colloid Interface Sci. 2013;397:73–9.
Hao L, Yang H, Lei ZL. Synthesis and properties of thermo-responsive macroporous PAM-co-PNIPAM microspheres. Mater Lett. 2012;70:83–5.
Talelli M, Rijcken CJF, Lammers T, Seevinck PR, Storm G, van Nostrum CF, et al. Superparamagnetic iron oxide nanoparticles encapsulated in biodegradable thermosensitive polymeric micelles: toward a targeted nanomedicine suitable for image-guided drug delivery. Langmuir. 2009;25:2060–7.
Gaharwar AK, Wong JE, Muller-Schulte D, Bahadur D, Richtering W. Magnetic nanoparticles encapsulated within a thermoresponsive polymer. J Nanosci Nanotechnol. 2009;9:5355–61.
Lyon JL, Fleming DA, Stone MB, Schiffer P, Williams ME. Synthesis of Fe oxide core/Au shell nanoparticles by iterative hydroxylamine seeding. Nano Lett. 2004;4:719–23.
Taratula O, Garbuzenko OB, Kirkpatrick P, Pandya I, Savla R, Pozharov VP, et al. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J Control Release. 2009;140:284–93.
Jun L, Bochu W, Yazhou W. Thermo-sensitive polymers for controlled-release drug delivery systems. Int J Pharmacol. 2006;2:513–9.
Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65:157–70.
Peng XH, Qian XM, Mao H, Wang AY, Chen Z, Nie SM, et al. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int J Nanomedicine. 2008;3:311–21.
Kievit FM, Wang FY, Fang C, Mok H, Wang K, Silber JR, et al. Doxorubicin loaded iron oxide nanoparticles overcome multidrug resistance in cancer in vitro. J Control Release. 2011;152:76–83.
Quan QM, Xie J, Gao HK, Yang M, Zhang F, Liu G, et al. HSA coated iron oxide nanoparticles as drug delivery vehicles for cancer therapy. Mol Pharm. 2011;8:1669–76.
Yang LL, Cao ZH, Sajja HK, Mao H, Wang LY, Geng HY, et al. Development of receptor targeted magnetic iron oxide nanoparticles for efficient drug delivery and tumor imaging. J Biomed Nanotechnol. 2008;4:439–49.
Taratula O, Dani RK, Schumann C, Xu H, Wang A, Song H, et al. Multifunctional nanomedicine platform for concurrent delivery of chemotherapeutic drugs and mild hyperthermia to ovarian cancer cells. Int J Pharm. 2013;458:169–80.
Hruby M, Konak C, Ulbrich K. Polymeric micellar pH-sensitive drug delivery system for doxorubicin. J Control Release. 2005;103:137–48.
MacKay JA, Chen MN, McDaniel JR, Liu WG, Simnick AJ, Chilkoti A. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat Mater. 2009;8:993–9.
Shi M, Ho K, Keating A, Shoichet MS. Doxorubicin-conjugated immuno-nanoparticles for intracellular anticancer drug delivery. Adv Funct Mater. 2009;19:1689–96.
Acknowledgments
This work was supported in part by the funding provided by the Medical Research Foundation of Oregon, PhRMA Foundation and the College of Pharmacy, Oregon State University. We thank Teresa Sawyer (Electron Microscopy Facility, OSU) for TEM analysis and Dr. Christine Pastorek (Department of Chemistry, OSU) for access to the FTIR-ATR and TGA instruments.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Guest Editors: Mahavir B. Chougule and Chalet Tan
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 285 kb)
Rights and permissions
About this article
Cite this article
Dani, R.K., Schumann, C., Taratula, O. et al. Temperature-Tunable Iron Oxide Nanoparticles for Remote-Controlled Drug Release. AAPS PharmSciTech 15, 963–972 (2014). https://doi.org/10.1208/s12249-014-0131-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0131-x