Abstract
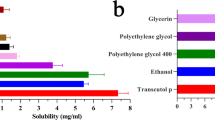
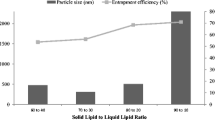
Meloxicam gel was designed based on the matching of the solubility parameter (δ) of the drug with that of the polymer and subsequently with skin for improved dermal delivery of meloxicam. The δ of meloxicam (11.48 (cal/cm3)0.5) determined by solubility measurement was matched statistically to the solubility parameter of monomers, n-vinyl-2-pyrrolidone, polyvinyl alcohol (PVA), hydroxyl ethyl methacrylate, ethylene glycol methacrylate (EGMA) determined by intrinsic viscosity measurement. Consequently gels were formulated by polymerization in selected solvent blend of water/ethyl acetate (20:80) in which the drug showed maximum solubility. Thus, F1–F16 formulations designed were evaluated for physicochemical properties, textural analysis, and in vitro drug release. On the basis of optimum characteristics, F2 (PVA, δ = 16.96 (cal/cm3)0.5) and F8 (EGMA, δ = 18.35 (cal/cm3)0.5) formulated by suspension polymerization were selected and subjected to skin irritation and topical anti-inflammatory studies. The formulation F8 demonstrated significant (p < 0.05) of anti-inflammatory activity in comparison to marketed piroxicam gel and was free from irritation.






Similar content being viewed by others
References
Brunton LL, Lazo Js, Parker KL. Goodman & Gilmans’s the pharmacological basis of therapeutics. 11th ed. NY: McGraw Hill Medical Publication Division; 2006.
Chang JS, Huang YB, Hou SS, Wang RJ, Wu PC, Tsai YH. Formulation optimization of meloxicam sodium gel using response surface methodology. Int J Pharm. 2007;339:48–54. doi:10.1016/j.ijpharm.2007.01.033.
Chang JS, Tsai YH, Wu PC, Huang YB. The effect of mixed-solvent and terpenes on percutaneous absorption of meloxicam gel. Drug Dev Ind Pharm. 2007;33:984–9. doi:10.1080/03639040601150294.
Wang Y, Chen M, Li X, Huang Y, Liang W. A hybrid thermosensitive chitosan gel for sustained release of meloxicam. J Biomater Sci Poly Ed. 2008;19:1239–47.
Tsai Y, Huang Y, Wu P, Chang J. In vitro evaluation of meloxicam permeation using response surface methodology. J Food Drug Anal. 2006;14:236–41.
Weiner ML, Kotkoskie LA. Excipient toxicity and safety. New York: Marcel Dekker; 2000.
Hancock BC, York P, Rowe RC. The use of solubility parameters in pharmaceutical dosage form design. Int J Pharm. 1997;148:1–21.
Sloan KB. The use of solubility parameters of drug and vehicle to describe skin transport in topical drug delivery formulation. New York: Marcel Dekker; 2000.
Romero S, Escalera B, Bustamante P. Solubility behaviour of polymorphs I and II of mefenamic acid in solvent mixtures. Int J Pharm. 1999;178:193–202. doi:10.1016/j.ijpharm.2006.04.020.
Barra J, Pena MA, Bustamanate P. Proposition of group molar constants for sodium to calculate the partial solubility parameters of sodium salt using the van Krevelen group contribution method. Eur J Pharm Sci. 1999;10:153–61. doi:10.1016/S0928-0987(00)00061-0.
Josyula VR, Karanth H. Studies on solubility parameter of amoxycillin trihydrate: influence on in vitro release and antibacterial activity. Ind J Pharm Sci. 2005;67:342–5.
Bustamante P, Lupion JN, Escalera B. A new method to determine the partial solubility parameters of polymers from intrinsic viscosity. Eur J Pharm Sci. 2004;24:229–37. doi:10.1016/S0378.5173 (99)00379-9.
Sen M, Uzun C, Güven O. Controlled release of terbinafine hydrochloride from pH sensitive poly(acrylamide/maleic acid) gels. Int J Pharm. 2000;203:149–57. doi:10.1016/S0378-5173(01)00804-3.
Zhang Y, Zhu W, Ding J. Preparation of thermo sensitive microgels via suspension polymerization using different temperature protocols. J Biomed Mater Res. 2005;75:342–9.
Fang J, Hwang T, Leu Y. Effect of enhancer and retarders on percutaneous absorption of flurbiprofen from gels. Int J Pharm. 2003;250:313–25. doi:10.1016/S0378-5173(02)00540-9.
Pourjavadi A, Kurdtabar M, Mahdavinia GR, Hosseinzadeh H. Synthesis and super-swelling behaviour of novel protein based superabsorbent gel. Poly Bull. 2006;57:813–24. doi:10.1016/j.carbpol.2008.12.019.
Singla AK, Pathak K. Topical anti-inflammatory effects of Euphorbia prostrata on carrageenan induced foot pad oedema in mice. J Ethnopharmacol. 1990;29:291–4.
Aulton ME. Pharmaceutics—the science of dosage form design. 2nd ed. New York: Churchill Livingstone; 2002.
Ghosh TK, Jasti BR. Theory and practice of contemporary pharmaceutics. Boca Raton: CRC; 2005.
Sen M, Guven O. Determination of solubility parameter of poly (N-vinyl 2-pyrrolidone/ethylene glycol dimethacrylate) gels by swelling measurement. J Polym Sci Part B: Poly Physics. 1997;36:213–9.
Malkin AY, Kulichikin SG. Rheokinetics of free-radical polymerization. Polymer. 1985;25:778–84.
Cioffi M, Ganzeveld KJ, Hoffmann AC, Janssen LPBM. A rheokinetic study of bulk free radical polymerization performed with a helical barrel rheometer. Polym Engg Sc. 2004;44:179–85.
Peng HT, Mok M, Martineau L, Shek PN. Gel—elastomer composite biomaterials: 2. Effects of aging methacrylated gelatin solutions on the preparation and physical properties of interpenetrating polymer networks. J Mater Sci: Mater in Med. 2007;18:1025–35.
Yosipovitch G, Hu J. The importance of skin pH and aging. CWI Medical. 2003;11:88–93.
Elvira C, Mano JF, Roman JS, Resis RL. Starch based biodegradable gels with potential biomedical application as drug delivery systems. Biomaterials. 2002;23:1955–66. doi:10.1016/S0142-9612(01)00322-2.
Morita R, Honda R, Takahashi Y. Development of oral controlled release preparation, a swelling controlled release system (SCRS). II. In vitro and in vivo evaluation. J Control Rel. 2000;68:115–20. doi:10.1016/S0168-3659(00)00244-3.
Isabel F, Alemeida M, Fernanda B. Evaluation of physical stability of two oleogels. Int J Pharm. 2006;327:73–7. doi:10.1016/j.ijpharm.2006.07.036.
Du Toit LC, Pillay V, Danckwerts MP. Application of synergism and variation in ionic compatibilities within a hydrophilic polymeric sodium starch glycolate-kappa-carrageenan combination: textural profiling of the suspension behavior. J Bioact Compat Pol. 2006;21:107–22.
Shastri D, Pandya H, Parikh RK, Patel CN. Smart gel in controlled drug delivery. Pharma Times. 2006;38:13–8.
Osborne DW, Amann HA. Topical drug delivery formulation. New York: Marcel Dekker; 2000.
Nugent MJD, Hanley A, Tomkins PT, Higginbotham CL. Investigation of a novel freeze–thaw process for the production of drug delivery gels. J Mat Sci. 2005;16:1149–58. doi:10.1002/app.27832.
Gowariker VR, Viswanatha NV, Sreedher J. Polymer science. New Delhi: New Age International; 2006.
Acknowledgment
The authors would like to thank Indian Institute of Technology, Roorkee for providing the facilities for SEM and DSC analysis of the samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, D., Pathak, K. Design, Characterization, and Evaluation of Meloxicam Gel Prepared by Suspension and Solution Polymerization Using Solubility Parameter as the Basis for Development. AAPS PharmSciTech 11, 133–142 (2010). https://doi.org/10.1208/s12249-009-9369-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-009-9369-0