Abstract
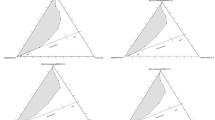
Formulation of a new oil-in-water (o/w) microemulsion composed of castor oil/Tween 80/ethanol/phosphate buffer for enhancing the loading capacity of an anti-inflammatory drug piroxicam has been accomplished. The pseudo-ternary phase diagram has been delineated at constant surfactant/cosurfactant ratio (1:2). The internal structure of so created four-component system was elucidated by means of an analysis of isotropic area magnitudes in the phase diagram. Conductivity (σ), kinematic viscosity (k η ), and surface tension (γ) studies with the variation in Φ w (weight fraction of aqueous phase) show the occurrence of structural changes from water-in-oil (w/o) microemulsion to oil-in-water (o/w). Along with the solubility and partition studies of piroxicam in microemulsion components, the changes in the microstructure of the microemulsion after incorporation of drug have been evaluated using pH, σ, γ, k η , and density studies. Piroxicam, a poorly water-soluble drug displayed high solubility (1.0%) in an optimum microemulsion formulation using ethanol (55.0%), Tween 80 (26.5%), castor oil (7.5%), and phosphate buffer (11.0%). The results have shown that the microemulsion remained stable after the incorporation of piroxicam. Fluorescence spectra analysis taking pyrene as fluorescent probe was performed, and the results showed that pyrene was completely solubilized in the oil phases of the bicontinuous microemulsions. The fluorescence spectrum of the model drug piroxicam was used to probe the intramicellar region of nonionic microemulsion. The results showed that the piroxicam was localized in the interfacial film of microemulsion systems more deeply in the palisade layer with ethanol as the cosurfactant.












Similar content being viewed by others
References
Lopes LB, Scarpa MV, Pereira NL, De Oliveira LC, Oliveira AG. Interaction of sodium diclofenac with freeze-dried soya phosphatidylcholine and unilamellar liposomes. Revista Brasileira de Ciencias Farmaceuticas/Brazilian Journal of Pharmaceutical Sciences. 2006;42(4):497–504.
Park ES, Cui Y, Yun BJ, Ko IJ, Chi SC. Transdermal delivery of piroxicam using microemulsions. Arch Pharmacal Res. 2005;28(2):243–8.
Yuan Y, Li SM, Mo FK, Zhong DF. Investigation of microemulsion system for transdermal delivery of meloxicam. Int J Pharm. 2006;321(1–2):117–23.
Sarciaux JM, Acar L, Sado PA. Using microemulsion formulations for oral drug delivery of therapeutic peptides. Int J Pharm. 1995;120(2):127–36.
Ritschel WA. Methods and findings in experimental and clinical pharmacology. 1991;13(3):205–20
Mehta SK, Kaur G, Bhasin KK. Analysis of Tween based microemulsion in the presence of TB drug rifampicin. Colloids Surf., B Biointerfaces. 2007;60(1):95–104.
Sebastien H, Devin VM, Mark GA, Mark RP. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998;87(8):922–5.
Sintov AC, Botner S. Transdermal drug delivery using microemulsion and aqueous systems: influence of skin storage conditions on the in vitro permeability of diclofenac from aqueous vehicle systems. Int J Pharm. 2006;311(1–2):55–62.
Spernath A, Aserin A. Microemulsions as carriers for drugs and nutraceuticals. Adv Colloid Interface Sci. 2006;128–130:47–64.
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45(1):89–121.
López A, Llinares F, Cortell C, Herráez M. Comparative enhancer effects of Span®20 with Tween®20 and Azone® on the in vitro percutaneous penetration of compounds with different lipophilicities. Int J Pharm. 2000;202(1–2):133–40.
Fang JY, Yu SY, Wu PC, Huang YB, Tsai YH. In vitro skin permeation of estradiol from various proniosome formulations. Int J Pharm. 2001;215(1–2):91–9.
Krauel K, Davies NM, Hook S, Rades T. Using different structure types of microemulsions for the preparation of poly(alkylcyanoacrylate) nanoparticles by interfacial polymerization. J Control Release. 2005;106(1–2):76–87.
Mehta SK, Kaur G, Bhasin KK. Incorporation of antitubercular drug isoniazid in pharmaceutically accepted microemulsion: effect on microstructure and physical parameters. Pharm Res. 2008;25(1):227–36.
He D, Yang C, Ma M, Zhuang L, Chen X, Chen S. Studies of the chemical properties of tri-n-octylamine-secondary octanol-kerosene-HCl-H2O microemulsions and its extraction characteristics for cadmium(II). Colloids Surf A. 2004;232(1):39–47.
Podlogar F, Gašperlin M, Tomšič M, Jamnik A, Rogač MB. Structural characterisation of water-Tween 40®/Imwitor 308®-isopropyl myristate microemulsions using different experimental methods. Int J Pharm. 2004;276(1–2):115–28.
Sripriya R, Muthu Raja K, Santhosh G, Chandrasekaran M, Noel M. The effect of structure of oil phase, surfactant and co-surfactant on the physicochemical and electrochemical properties of bicontinuous microemulsion. J Colloid Interface Sci. 2007;314(2):712–7.
Date AA, Nagarsenker MS. Parenteral microemulsions: an overview. Int J Pharm. 2008;355(1–2):19–30.
Pershing LK, Parry GE, Lambert LD. Disparity of in vitro and in vivo oleic acid-enhanced beta-estradiol percutaneous absorption across human skin. Pharm Res. 1993;10:1745–50.
Tanojo H, Junginger HE, Boddé HE. In vivo human skin permeability enhancement by oleic acid: transepidermal water loss and Fourier-transform infrared spectroscopy studies. J Control Release. 1997;47(1):31–9.
Peltola S, Saarinen-Savolainen P, Kiesvaara J, Suhonen TM, Urtti A. Microemulsions for topical delivery of estradiol. Int J Pharm. 2003;254(2):99–107.
Subramanian N, Ray S, Ghosal SK, Bhadra R, Moulik SP. Formulation design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxib. Biol Pharm Bull. 2004;27(12):1993–9.
Lv FF, Zheng LQ, Tung CH. Phase behavior of the microemulsions and the stability of the chloramphenicol in the microemulsion-based ocular drug delivery system. Int J Pharm. 2005;301(1–2):237–46.
Fanun M. Water solubilization in mixed nonionic surfactants microemulsions. J Dispers Sci Technol. 2008;29(8):1043–52.
Alany RG, Rades T, Agatonovic-Kustrin S, Davies NM, Tucker IG. Effects of alcohols and diols on the phase behaviour of quaternary systems. Int J Pharm. 2000;196(2):141–5.
Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006;123–126:369–85.
Grest GS, Webman I, Safran SA, Bug ALR. Dynamic percolation in microemulsions. Phys Rev A. 1986;33(4):2842.
Mehta SK, Bala K. Tween-based microemulsions: a percolation view. Fluid Phase Equilib. 2000;172(2):197–209.
Dasilva-Carbalhal J, Garcia-Rio L, Gomez-Diaz D, Mejuto JC, Perez-Lorenzo M. Influence of glymes upon percolative phenomena in AOT-based microemulsions. J Colloid Interface Sci. 2005;292(2):591–4.
Podlogar F, Bester Rogac M, Gasperlin M. The effect of internal structure of selected water–Tween 40®–Imwitor 308®–IPM microemulsions on ketoprofene release. Int J Pharm. 2005;302(1–2):68–77.
Kumar P, Mittal KL. Handbook of microemulsion science and technology. Boca Raton: CRC; 1999. p. 357–86.
Patzold G, Dawson K. Rheology of self-assembled fluids. J Chem Phys. 1996;104(15):5932–41.
Djordjevic L, Primorac M, Stupar M, Krajisnik D. Characterization of caprylocaproyl macrogolglycerides based microemulsion drug delivery vehicles for an amphiphilic drug. Int J Pharm. 2004;271(1–2):11–9.
Mitra RK, Paul BK. Physicochemical investigations of microemulsification of eucalyptus oil and water using mixed surfactants (AOT+Brij-35) and butanol. J Colloid Interface Sci. 2005;283(2):565–77.
Leser ME, van Evert WC, Agterol WGM. Phase behaviour of lecithin–water–alcohol–triacylglycerol mixtures. Colloids Surf A. 1996;116(3):293–308.
Ding L, Dominska M, Fang Y, Blanchard GJ. Fluorescence and electrochemistry studies of pyrene-functionalized surface adlayers to probe the microenvironment formed by cholesterol. Electrochim Acta. 2008;53(23):6704–13.
ZielinÌska K, Wilk KA, Jezierski A, Jesionowski T. Microstructure and structural transition in microemulsions stabilized by aldonamide-type surfactants. J Colloid Interface Sci. 2008;321(2):408–17.
Lianos P. Fluorescence probe study of the interaction between pyrene and microemulsion-polymerized styrene. J Phys Chem. 1982;86(11):1935–7.
Kalyanasundaram K, Thomas JK. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc. 1977;99(7):2039–44.
Zhang S, Rusling JF. Evaluation of microemulsions of cationic surfactants and a polyoxyethylene cosurfactant for electrolytic dechlorinations of chlorobiphenyls. J Colloid Interface Sci. 1996;182(2):558–63.
Songca SP, Mbatha B. Solubilization of meso-tetraphenylporphyrin photosensitizers by substitution with fluorine and with 2, 3-dihydroxy-1-propyloxy groups. J Pharm Pharmacol. 2000;52:1361–7.
Khan AM, Shah SS. A UV–visible study of partitioning of pyrene in an anionic surfactant sodium dodecyl sulfate. J Dispers Sci Technol. 2008;29(10):1401–7.
Acknowledgments
The financial support of Quaid-i-Azam University and Higher Education Commission of Pakistan is duly acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Nazar, M.F., Khan, A.M. & Shah, S.S. Microemulsion System with Improved Loading of Piroxicam: A Study of Microstructure. AAPS PharmSciTech 10, 1286–1294 (2009). https://doi.org/10.1208/s12249-009-9328-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-009-9328-9