Abstract
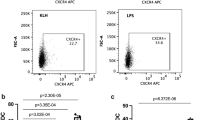
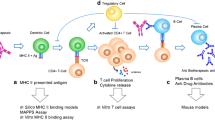
Validation of key analytical and functional performance characteristics of in vitro immunogenicity risk assessment assays increases our confidence in utilizing them for screening biotherapeutics. Herein, we present a fit-for-purpose (FFP) validation of a dendritic cell (DC) activation assay designed to assess the immunogenicity liability of protein biotherapeutics. Characterization of key assay parameters was achieved using monocyte-derived DCs (MoDCs) treated with cell culture medium only (i.e., background control (BC)), keyhole limpet hemocyanin (KLH) as system positive control (SPC), and 2 therapeutic monoclonal antibodies (mAbs) with known clinical immunogenicity profiles (bococizumab and TAM163) as therapeutic controls (TCs). In the absence of established validation guidelines for primary cell-based assays, the present DC activation assay was validated using a novel FFP approach which allows more flexibility in selection of validation parameters and designing of experiments based on the intended use of the assay. The present FFP validation allowed us to understand the impact of experimental variables on assay precision, develop a clear concise readout for DC activation results, establish a reliable response threshold to define a result as a positive DC activation response, and define in-study donor acceptance criteria and cohort size. FFP validation of this DC activation assay indicated that the assay is sufficient to support its context of use, a preclinical immunogenicity risk management tool.





Similar content being viewed by others
References
U.S. Food and Drug Administration/Center for Drug Evaluation and Research. Guidance for industry: immunogenicity assessment for therapeutic protein products. Forms based on FDA Drugs guidence. 2014.
Lamberth K, Reedtz-Runge SL, Simon J, Klementyeva K, Pandey GS, Padkjaer SB, et al. Post hoc assessment of the immunogenicity of bioengineered factor VIIa demonstrates the use of preclinical tools. Sci. Transl. Med. 2017;9(372).
Mazor R, Onda M, Pastan I. Immunogenicity of therapeutic recombinant immunotoxins. Immunol Rev. 2016;270(1):152–64.
Mazor R, Zhang J, Xiang L, Addissie S, Awuah P, Beers R, et al. Recombinant immunotoxin with T-cell epitope mutations that greatly reduce immunogenicity for treatment of mesothelin-expressing tumors. Mol. Cancer Ther. 2015;14(12):2789–96.
Delluc S, Ravot G, Maillere B. Quantitative analysis of the CD4 T-cell repertoire specific to therapeutic antibodies in healthy donors. FASEB J. 2011;25(6):2040–8.
Joubert MK, Deshpande M, Yang J, Reynolds H, Bryson C, Fogg M, et al. Use of in vitro assays to assess immunogenicity risk of antibody-based biotherapeutics. PLoS One. 2016;11(8):e0159328.
Vaughan K, Xu X, Caron E, Peters B, Sette A. Deciphering the MHC-associated peptidome: a review of naturally processed ligand data. Expert Rev Proteom. 2017;14(9):729–36.
Wullner D, Zhou L, Bramhall E, Kuck A, Goletz TJ, Swanson S, et al. Considerations for optimization and validation of an in vitro PBMC derived T cell assay for immunogenicity prediction of biotherapeutics. Clin Immunol. 2010;137(1):5–14.
Xue L, Hickling T, Song R, Nowak J, Rup B. Contribution of enhanced engagement of antigen presentation machinery to the clinical immunogenicity of a human interleukin (IL)-21 receptor-blocking therapeutic antibody. Clin. Exp. Immunol. 2016;183(1):102–13.
Rombach-Riegraf V, Karle AC, Wolf B, Sordé L, Koepke S, Gottlieb S, et al. Aggregation of human recombinant monoclonal antibodies influences the capacity of dendritic cells to stimulate adaptive T-cell responses in vitro. PLoS One. 2014;9(1).
Walsh RE, Lannan M, Wen Y, Wang X, Moreland CA, Willency J, et al. Post-hoc assessment of the immunogenicity of three antibodies reveals distinct immune stimulatory mechanisms. mAbs. 2020;12(1):1764829.
ICH Expert Working Group. Validation of analytical procedures: text and methodology Q2 (R1).pdf>. 2005.
Lee JW, Devanarayan V, Barrett YC, Weiner R, Allinson J, Fountain S, et al. Fit-for-purpose method development and validation for successful biomarker measurement. Pharm. Res. 2006;23(2):312–28.
Piccoli S.P SJM, Biomarker Assay Collaborative Evidentiary Considerations Writing Group; Critical Path Institute (C-Path). Points to Consider Document: Scientific and Regulatory Considerations for the Analytical Validation of Assays Used in the Qualification of Biomarkers in Biological Matrices. 2019. Available from: https://c-path.org/wp-content/uploads/2019/06/EvidConsid-WhitePaper-AnalyticalSectionV20190621.pdf.
U.S. Food and Drug Adminstration/Center for Drug Evaluation and Research. Biomarker Qualification: Evidentiary Framework: Guidence for Industry and FDA Staff. 2018.
Ridker PM, Tardif JC, Amarenco P, Duggan W, Glynn RJ, Jukema JW, et al. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N Engl J Med. 2017;376(16):1517–26.
Cummings J, Ward TH, Greystoke A, Ranson M, Dive C. Biomarker method validation in anticancer drug development. Br. J. Pharmacol. 2008;153(4):646–56.
Korber N, Behrends U, Hapfelmeier A, Protzer U, Bauer T. Validation of an IFNgamma/IL2 FluoroSpot assay for clinical trial monitoring. J Transl Med. 2016;14(1):175.
Maecker HT, Hassler J, Payne JK, Summers A, Comatas K, Ghanayem M, et al. Precision and linearity targets for validation of an IFNgamma ELISPOT, cytokine flow cytometry, and tetramer assay using CMV peptides. BMC Immunol. 2008;9:9.
O'Hara DM, Xu Y, Liang Z, Reddy MP, Wu DY, Litwin V. Recommendations for the validation of flow cytometric testing during drug development: II assays. J. Immunol. Methods. 2011;363(2):120–34.
Wu DY, Patti-Diaz L, Hill CG. Development and validation of flow cytometry methods for pharmacodynamic clinical biomarkers. Bioanalysis. 2010;2(9):1617–26.
Perfetto SP, Ambrozak D, Nguyen R, Chattopadhyay P, Roederer M. Quality assurance for polychromatic flow cytometry. Nat. Protoc. 2006;1(3):1522–30.
Wood JC. Establishing and maintaining system linearity. Current protocols in cytometry. 2001;Chapter 1:Unit 1.4.
Wood JC, Hoffman RA. Evaluating fluorescence sensitivity on flow cytometers: an overview. Cytometry. 1998;33(2):256–9.
Cunliffe J, Derbyshire N, Keeler S, Coldwell R. An approach to the validation of flow cytometry methods. Pharm. Res. 2009;26(12):2551–7.
Efron B, Tibshirani RJ. An introduction to the bootstrap. New York: Chapman & Hall; 1993.
Burdick RKB, Connie M Montgomery DC. Design and analysis of gauge R&R studies: making decisions with confidence intervals in random and mixed ANOVA models: SIAM; 2005.
Montgomery DC. Introduction to statistical quality control. 7 ed: John Wiley and Sons; 2013.
Ito S, Ikuno T, Mishima M, Yano M, Hara T, Kuramochi T, et al. In vitro human helper T-cell assay to screen antibody drug candidates for immunogenicity. J Immunotoxicol. 2019;16(1):125–32.
Jawa V, Joubert MK, Zhang Q, Deshpande M, Hapuarachchi S, Hall MP, et al. Evaluating immunogenicity risk due to host cell protein impurities in antibody-based biotherapeutics. AAPS J. 2016;18(6):1439–52.
Morgan H, Tseng SY, Gallais Y, Leineweber M, Buchmann P, Riccardi S, et al. Evaluation of in vitro assays to assess the modulation of dendritic cells functions by therapeutic antibodies and aggregates. Front Immunol. 2019;10:601.
Jawa V, Cousens LP, Awwad M, Wakshull E, Kropshofer H, De Groot AS. T-cell dependent immunogenicity of protein therapeutics: preclinical assessment and mitigation. Clin Immunol. 2013;149(3):534–55.
Moussa EM, Panchal JP, Moorthy BS, Blum JS, Joubert MK, Narhi LO, et al. Immunogenicity of therapeutic protein aggregates. J. Pharm. Sci. 2016;105(2):417–30.
Acknowledgments
The authors thank Timothy Hickling and Ying Wang for support and consultation for the FFP validation planning and data review. We also thank Mahwish Natalia for technical assistance during assay development and pre-FFP validation studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Wickramarachchi, D., Steeno, G., You, Z. et al. Fit-for-Purpose Validation and Establishment of Assay Acceptance and Reporting Criteria of Dendritic Cell Activation Assay Contributing to the Assessment of Immunogenicity Risk. AAPS J 22, 114 (2020). https://doi.org/10.1208/s12248-020-00491-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-00491-8