Abstract
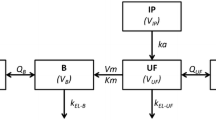
Intraperitoneal chemoperfusion (IPEC) of cisplatin is a popular treatment for advanced ovarian cancer, typically under hyperthermia (HIPEC). The use of cisplatin under (H)IPEC is off-label, and the role of hyperthermia is unknown. The aim of this study was to characterize the pharmacokinetic/pharmacodynamic (PKPD) properties of cisplatin under (H)IPEC and to predict the optimal treatment regimen. Using a randomized design, data on intact cisplatin perfusate and plasma concentrations, leukocyte counts—a hematotoxicity marker—and serum creatinine—a nephrotoxicity marker—were collected from 50 patients treated with a combination of cytoreductive surgery (CRS) and either normothermic or hyperthermic IPEC of cisplatin dosed at 75, 100, and 120 mg/m2. The non-linear mixed effects modeling technique was used to construct the PKPD models. The PK of intact cisplatin was characterized by a two-compartment model. A semi-physiological myelosuppression model for the leukopenia was modified to account for the CRS-induced leukocytosis and the residual myelosuppression effect of neoadjuvant chemotherapy. The incidence and severity of nephrotoxicity were described by a discrete-time Markov model. Hyperthermia increased the absorption rate of cisplatin by 16.3% but did not show a clinically relevant impact on the investigated toxicities compared with normothermia. Leukopenia was not severe, but nephrotoxicity can become severe or life-threatening and was affected by the dose and IPEC duration. The model predicted that nephrotoxicity is minimal at a cisplatin dose of 75 mg/m2 with an IPEC duration of 1–2 h and an 1-h duration is favored for doses between 100 and 120 mg/m2.

Graphical abstract





Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
de Bree E, Helm CW. Hyperthermic intraperitoneal chemotherapy in ovarian cancer: rationale and clinical data. Expert Rev Anticancer Ther. 2012;12(7):895–911.
Tummala MK, Alagarsamy S, McGuire WP. Intraperitoneal chemotherapy: standard of care for patients with minimal residual stage III ovarian cancer? Expert Rev Anticancer Ther. 2008;8(7):1135–47.
Polom K, Roviello G, Generali D, Marano L, Petrioli R, Marsili S, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for treatment of ovarian cancer. Int J Hyperth. 2016;32(3):298–310.
Van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HW, Hermans RH, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–40.
Van Driel WJ, Lok CA, Verwaal V, Sonke GS. The role of hyperthermic intraperitoneal intraoperative chemotherapy in ovarian cancer. Curr Treat Options in Oncol. 2015;16(4):14.
Ceelen WP, Van Nieuwenhove Y, Van Belle S, Denys H, Pattyn P. Cytoreduction and hyperthermic intraperitoneal chemoperfusion in women with heavily pretreated recurrent ovarian cancer. Ann Surg Oncol. 2012;19(7):2352–9.
Cowan RA, O’Cearbhaill RE, Zivanovic O, Chi DS. Current status and future prospects of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) clinical trials in ovarian cancer. Int J Hyperth. 2017;33(5):548–53.
de Bree E, Theodoropoulos PA, Rosing H, Michalakis J, Romanos J, Beijnen JH, et al. Treatment of ovarian cancer using intraperitoneal chemotherapy with taxanes: from laboratory bench to bedside. Cancer Treat Rev. 2006;32(6):471–82.
Fujiwara K, Armstrong D, Morgan M, Markman M. Principles and practice of intraperitoneal chemotherapy for ovarian cancer. Int J Gynecol Cancer. 2007;17(1):1–20.
Hettinga J, Konings A, Kampinga H. Reduction of cellular cisplatin resistance by hyperthermia—a review. Int J Hyperth. 1997;13(5):439–57.
Takemoto M, Kuroda M, Urano M, Nishimura Y, Kawasaki S, Kato H, et al. The effect of various chemotherapeutic agents given with mild hyperthermia on different types of tumours. Int J Hyperth. 2003;19(2):193–203.
Sugarbaker PH. Peritonectomy procedures. Surg Oncol Clin N Am. 2003;12(3):703–27.
Hanada K, Nishijima K, Ogata H, Atagi S, Kawahara M. Population pharmacokinetic analysis of cisplatin and its metabolites in cancer patients: possible misinterpretation of covariates for pharmacokinetic parameters calculated from the concentrations of unchanged cisplatin, ultrafiltered platinum and total platinum. Jpn J Clin Oncol. 2001;31(5):179–84.
Farris FF, King FG, Dedrick RL, Litterst CL. Physiological model for the pharmacokinetics ofcis-dichlorodiammineplatinum (II)(DDP) in the tumored rat. J Pharmacokinet Biopharm. 1985;13(1):13–39.
Alderden RA, Hall MD, Hambley TW. The discovery and development of cisplatin. J Chem Educ. 2006;83(5):728.
Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334(2):115–24.
Barabas K, Milner R, Lurie D, Adin C. Cisplatin: a review of toxicities and therapeutic applications. Vet Comp Oncol. 2008;6(1):1–18.
Reichman TW, Cracchiolo B, Sama J, Bryan M, Harrison J, Pliner L, et al. Cytoreductive surgery and intraoperative hyperthermic chemoperfusion for advanced ovarian carcinoma. J Surg Oncol. 2005;90(2):51–6.
Königsrainer I, Horvath P, Struller F, Grischke EM, Wallwiener D, Königsrainer A, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in recurrent epithelial ovarian cancer with peritoneal metastases: a single centre experience. Langenbeck's Arch Surg. 2014;399(5):589–94.
Batista TP, Badiglian Filho L, Leão CS. Exploring flow rate selection in HIPEC procedures. Rev Col Bras Cir. 2016;43(6):476–9.
Campbell AB, Kalman SM, Jacobs C. Plasma platinum levels: relationship to cisplatin dose and nephrotoxicity. Cancer Treat Rep. 1983;67(2):169–72.
Reece PA, Stafford I, Russell J, Khan M, Gill P. Creatinine clearance as a predictor of ultrafilterable platinum disposition in cancer patients treated with cisplatin: relationship between peak ultrafilterable platinum plasma levels and nephrotoxicity. J Clin Oncol. 1987;5(2):304–9.
Klaver YL, Hendriks T, Lomme RM, Rutten HJ, Bleichrodt RP, de Hingh IH. Hyperthermia and intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis: an experimental study. Ann Surg. 2011;254(1):125–30.
Carlier C, Laforce B, Van Malderen SJ, Gremonprez F, Tucoulou R, Villanova J, et al. Nanoscopic tumor tissue distribution of platinum after intraperitoneal administration in a xenograft model of ovarian cancer. J Pharm Biomed Anal. 2016;131:256–62.
Alvovsky I, Bespalov V, Kireeva G. Cisplatin in NIPEC or HIPEC? Ann Surg. 2018;29(suppl_8):mdy268.020.
Xie F, Colin P, Van Bocxlaer J. Zwitterionic hydrophilic interaction liquid chromatography-tandem mass spectrometry with HybridSPE-precipitation for the determination of intact cisplatin in human plasma. Talanta. 2017;174:171–8.
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20(24):4713–21.
Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31(8):318–24.
Pérez-Ruixo C, Valenzuela B, Peris JE, Bretcha-Boix P, Escudero-Ortiz V, Farré-Alegre J, et al. Neutrophil dynamics in peritoneal carcinomatosis patients treated with cytoreductive surgery and hyperthermic intraperitoneal oxaliplatin. Clin Pharmacokinet. 2013;52(12):1111–25.
Cotte E, Colomban O, Guitton J, Tranchand B, Bakrin N, Gilly FN, et al. Population pharmacokinetics and pharmacodynamics of cisplatinum during hyperthermic intraperitoneal chemotherapy using a closed abdominal procedure. J Clin Pharmacol. 2011;51(1):9–18.
Royer B, Jullien V, Guardiola E, Heyd B, Chauffert B, Kantelip JP, et al. Population pharmacokinetics and dosing recommendations for cisplatin during intraperitoneal peroperative administration: development of a limited sampling strategy for toxicity risk assessment. Clin Pharmacokinet. 2009;48(3):169–80.
Royer B, Kalbacher E, Onteniente S, Jullien V, Montange D, Piedoux S, et al. Intraperitoneal clearance as a potential biomarker of cisplatin after intraperitoneal perioperative chemotherapy: a population pharmacokinetic study. Br J Cancer. 2012;106(3):460–7.
Cashin PH, Ehrsson H, Wallin I, Nygren P, Mahteme H. Pharmacokinetics of cisplatin during hyperthermic intraperitoneal treatment of peritoneal carcinomatosis. Eur J Clin Pharmacol. 2013;69(3):533–40.
Urien S, Lokiec F. Population pharmacokinetics of total and unbound plasma cisplatin in adult patients. Br J Clin Pharmacol. 2004;57(6):756–63.
Royer B, Guardiola E, Polycarpe E, Hoizey G, Delroeux D, Combe M, et al. Serum and intraperitoneal pharmacokinetics of cisplatin within intraoperative intraperitoneal chemotherapy: influence of protein binding. Anti-Cancer Drugs. 2005;16(9):1009–16.
O'dwyer PJ, Stevenson JP, Johnson SW. Clinical pharmacokinetics and administration of established platinum drugs. Drugs. 2000;59(4):19–27.
Borbás E, Sinkó B, Tsinman O, Tsinman K, Ev K, Démuth B, et al. Investigation and mathematical description of the real driving force of passive transport of drug molecules from supersaturated solutions. Mol Pharm. 2016;13(11):3816–26.
Specenier PM, Ciuleanu T, Latz JE, Musib LC, Darstein CL, Vermorken JB. Pharmacokinetic evaluation of platinum derived from cisplatin administered alone and with pemetrexed in head and neck cancer patients. Cancer Chemother Pharmacol. 2009;64(2):233–41.
Burdon PC, Martin C, Rankin SM. Migration across the sinusoidal endothelium regulates neutrophil mobilization in response to ELR+ CXC chemokines. Br J Haematol. 2008;142(1):100–8.
Evans ND, Cheung SA, Yates JW. Structural identifiability for mathematical pharmacology: models of myelosuppression. J Pharmacokinet Pharmacodyn. 2018;45(1):79–90.
Joerger M, Huitema AD, Richel DJ, Dittrich C, Pavlidis N, Briasoulis E, et al. Population pharmacokinetics and pharmacodynamics of paclitaxel and carboplatin in ovarian cancer patients: a study by the European organization for research and treatment of cancer-pharmacology and molecular mechanisms group and new drug development group. Clin Cancer Res. 2007;13(21):6410–8.
Hénin E, You B, VanCutsem E, Hoff P, Cassidy J, Twelves C, et al. A dynamic model of hand-and-foot syndrome in patients receiving capecitabine. Clin Pharmacol Ther. 2009;85(4):418–25.
Schindler E, Karlsson MO. A minimal continuous-time Markov pharmacometric model. AAPS J. 2017;19(5):1424–35.
Sheiner LB. A new approach to the analysis of analgesic drug trials, illustrated with bromfenac data. Clin Pharmacol Ther. 1994;56(3):309–22.
Zingmark P-H, Kågedal M, Karlsson MO. Modelling a spontaneously reported side effect by use of a Markov mixed-effects model. J Pharmacokinet Pharmacodyn. 2005;32(2):261–81.
Nagai N, Kinoshita M, Ogata H, Tsujino D, Wada Y, Someya K, et al. Relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity after intravenous infusions of cisplatin to cancer patients. Cancer Chemother Pharmacol. 1996;39(1–2):131–7.
Nagai N, Ogata H. Quantitative relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity in rats: importance of area under the concentration-time curve (AUC) as the major toxicodynamic determinant in vivo. Cancer Chemother Pharmacol. 1997;40(1):11–8.
Hanada K, Asano K, Nishimura T, Chimata T, Matsuo Y, Tsuchiya M, et al. Use of a toxicity factor to explain differences in nephrotoxicity and myelosuppression among the platinum antitumour derivatives cisplatin, carboplatin and nedaplatin in rats. J Pharm Pharmacol. 2008;60(3):317–22.
Le Saux O, Decullier E, Freyer G, Glehen O, Bakrin N. Long-term survival in patients with epithelial ovarian cancer following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Int J Hyperth. 2018;35(1):652–7.
Acknowledgments
The authors thank all study participants and their families. We also thank the clinical study team members at the different study sites.
Funding
This work was supported by the Fund for Scientific Research – Flanders (FWO) (project number: G016915N).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
An Vermeulen is an employee of Johnson & Johnson and holds stock/stock options in the company. She is also a visiting Professor at Ghent University. Wim Ceelen is a senior clinical researcher from the Fund for Scientific Research – Flanders (FWO). The other authors have no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, F., Van Bocxlaer, J., Colin, P. et al. PKPD Modeling and Dosing Considerations in Advanced Ovarian Cancer Patients Treated with Cisplatin-Based Intraoperative Intraperitoneal Chemotherapy. AAPS J 22, 96 (2020). https://doi.org/10.1208/s12248-020-00489-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-00489-2