Abstract
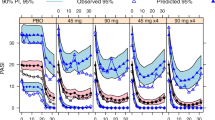
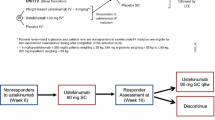
Disease status is often measured with bounded outcome scores (BOS) which report a discrete set of values on a finite range. The distribution of such data is often non-standard, such as J- or U-shaped, for which standard analysis methods assuming normal distribution become inappropriate. Most BOS analysis methods aim to either predict the data within its natural range or accommodate data skewness, but not both. In addition, a frequent modeling objective is to predict clinical response of treatment using derived disease endpoints, defined as meeting certain criteria of improvement from baseline in disease status. This objective has not yet been addressed in existing BOS data analyses. This manuscript compares a recently proposed beta distribution–based approach with the standard continuous analysis approach, using an established mechanism-based longitudinal exposure-response model to analyze data from two phase 3 clinical studies in psoriatic patients. The beta distribution–based approach is shown to be superior in describing the BOS data and in predicting the derived endpoints, along with predicting the response time course of a highly sensitive subpopulation.






Similar content being viewed by others
References
Overgaard RV, Ingwersen SH, Tornoe CW. Establishing good practices for exposure-response analysis of clinical endpoints in drug development. CPT Pharmacometrics Syst Pharmacol. 2015;4(10):565–75.
Lesaffre E, Rizopoulos D, Tsonaka R. The logistic transform for bounded outcome scores. Biostatistics. 2007;8(1):72–85.
Zhou H, Hu C, Zhu Y, Lu M, Liao S, Yeilding N, et al. Population-based exposure-efficacy modeling of ustekinumab in patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2010;50(3):257–67.
Hutmacher MM, French JL, Krishnaswami S, Menon S. Estimating transformations for repeated measures modeling of continuous bounded outcome data. Stat Med. 2011;30(9):935–49.
Smithson M, Verkuilen J. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol Methods. 2006;11(1):54–71.
Hu C, Adedokun OJ, Zhang L, Sharma A, Zhou H. Modeling near-continuous clinical endpoint as categorical: application to longitudinal exposure-response modeling of Mayo scores for golimumab in patients with ulcerative colitis. J Pharmacokinet Pharmacodyn. 2018;45(6):803–16.
Hu C, Randazzo B, Sharma A, Zhou H. Improvement in latent variable indirect response modeling of multiple categorical clinical endpoints: application to modeling of guselkumab treatment effects in psoriatic patients. J Pharmacokinet Pharmacodyn. 2017;44(5):437–48.
Hu C, Wasfi Y, Zhuang Y, Zhou H. Information contributed by meta-analysis in exposure-response modeling: application to phase 2 dose selection of guselkumab in patients with moderate-to-severe psoriasis. J Pharmacokinet Pharmacodyn. 2014;41(3):239–50.
Salinger DH, Endres CJ, Martin DA, Gibbs MA. A semi-mechanistic model to characterize the pharmacokinetics and pharmacodynamics of brodalumab in healthy volunteers and subjects with psoriasis in a first-in-human single ascending dose study. Clin Pharmacol Drug Dev. 2014;3(4):276–83.
Tham LS, Tang CC, Choi SL, Satterwhite JH, Cameron GS, Banerjee S. Population exposure-response model to support dosing evaluation of ixekizumab in patients with chronic plaque psoriasis. J Clin Pharmacol. 2014;54(10):1117–24.
Hu C, Yao Z, Chen Y, Randazzo B, Zhang L, Xu Z, et al. A comprehensive evaluation of exposure-response relationships in clinical trials: application to support guselkumab dose selection for patients with psoriasis. J Pharmacokinet Pharmacodyn. 2018;45(4):523–35.
Hu C. Exposure-response modeling of clinical end points using latent variable indirect response models. CPT Pharmacometrics Syst Pharmacol. 2014;3:e117.
Hu C, Adedokun OJ, Chen Y, Szapary PO, Gasink C, Sharma A, et al. Challenges in longitudinal exposure-response modeling of data from complex study designs: a case study of modeling CDAI score for ustekinumab in patients with Crohn’s disease. J Pharmacokinet Pharmacodyn. 2017;44(5):425–36.
Hu C. On the comparison of methods in analyzing bounded outcome score data. AAPS J. 2019;21(6):102.
Ursino M, Gasparini M. A new parsimonious model for ordinal longitudinal data with application to subjective evaluations of a gastrointestinal disease. Stat Methods Med Res. 2018;27(5):1376–93.
Hu C, Zhou H. Improvement in latent variable indirect response joint modeling of a continuous and a categorical clinical endpoint in rheumatoid arthritis. J Pharmacokinet Pharmacodyn. 2016;43(1):45–54.
Hu C, Xu Y, Zhuang Y, Hsu B, Sharma A, Xu Z, et al. Joint longitudinal model development: application to exposure-response modeling of ACR and DAS scores in rheumatoid arthritis patients treated with sirukumab. J Pharmacokinet Pharmacodyn. 2018;45(5):679–91.
Blauvelt A, Papp KA, Griffiths CE, Randazzo B, Wasfi Y, Shen YK, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–17.
Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–31.
Blauvelt A, Ferris LK, Yamauchi PS, Qureshi A, Leonardi CL, Farahi K, et al. Extension of ustekinumab maintenance dosing interval in moderate-to-severe psoriasis: results of a phase IIIb, randomized, double-blinded, active-controlled, multicentre study (PSTELLAR). Br J Dermatol. 2017;177(6):1552–61.
Hu C, Zhou H. An improved approach for confirmatory phase III population pharmacokinetic analysis. J Clin Pharmacol. 2008;48(7):812–22.
Hu C, Zhang J, Zhou H. Confirmatory analysis for phase III population pharmacokinetics. Pharm Stat. 2011;10(1):14–26.
Yao Z, Hu C, Zhu Y, Xu Z, Randazzo B, Wasfi Y, et al. Population pharmacokinetic modeling of guselkumab, a human IgG1lambda monoclonal antibody targeting IL-23, in patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58(5):613–27.
Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102(9):2941–52.
Sharma A, Jusko WJ. Characterization of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm. 1996;24(6):611–35.
Holford N. Clinical pharmacology = disease progression + drug action. Br J Clin Pharmacol. 2015;79(1):18–27.
Hu C, Szapary PO, Mendelsohn AM, Zhou H. Latent variable indirect response joint modeling of a continuous and a categorical clinical endpoint. J Pharmacokinet Pharmacodyn. 2014;41(4):335–49.
Hu C, Xu Z, Mendelsohn A, Zhou H. Latent variable indirect response modeling of categorical endpoints representing change from baseline. J Pharmacokinet Pharmacodyn. 2013;40(1):81–91.
Zhang L, Beal SL, Sheiner LB. Simultaneous vs. sequential analysis for population PK/PD data I: best-case performance. J Pharmacokinet Pharmacodyn. 2003;30(6):387–404.
Beal SL, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM user’s guides (1989–2009). Ellicott City: Icon Development Solutions; 2009.
Bauer RJ. NONMEM tutorial part II: estimation methods and advanced examples. CPT Pharmacometrics Syst Pharmacol. 2019.
Karlsson MO, Holford NHG. A tutorial on visual predictive checks 2008 [updated] 2008. Available from: www.page-meeting.org/?abstract=1434. Accessed 27 Feb 2020.
Hutmacher MM, French JL. Extending the latent variable model for extra correlated longitudinal dichotomous responses. J Pharmacokinet Pharmacodyn. 2011;38:833–59.
Liu Q, Shepherd BE, Li C, Harrell FE Jr. Modeling continuous response variables using ordinal regression. Stat Med. 2017;36(27):4316–35.
Sofen H, Smith S, Matheson RT, Leonardi CL, Calderon C, Brodmerkel C, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133(4):1032–40.
Hu C, Szapary PO, Yeilding N, Zhou H. Informative dropout modeling of longitudinal ordered categorical data and model validation: application to exposure-response modeling of physician’s global assessment score for ustekinumab in patients with psoriasis. J Pharmacokinet Pharmacodyn. 2011;38(2):237–60.
Vu TC, Nutt JG, Holford NH. Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment. Br J Clin Pharmacol. 2012;74(2):267–83.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Funding
This research was funded by the Janssen Research and Development, LLC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, C., Zhou, H. & Sharma, A. Applying Beta Distribution in Analyzing Bounded Outcome Score Data. AAPS J 22, 61 (2020). https://doi.org/10.1208/s12248-020-00441-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-00441-4