Abstract
Introduction
In this paper, we studied the effect over time of agomelatine, an antidepressant drug administered in patient with major depressive disorder, through item response theory (IRT), taking into account a strong placebo effect and missing not at random. We also assessed the informativeness of the HAMD-17 scale’s item.
Materials and Methods
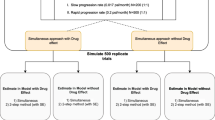
The data includes five phase III clinical trials sponsored by Servier Institute, totalling 1549 patients followed during a maximum of 1 year. At each observation, individual scores for the 17 items of the HAMD scale were recorded. The probability for each score was modelled with IRT. A non-linear mixed effects model was used to describe the evolution of the disease and was coupled with a time to event model to predict dropout. Clinical trial simulations were then used to compare placebo and active treatment. Informativeness of each item was evaluated using the Fisher information theory.
Results
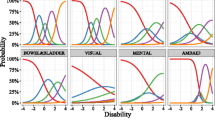
The best model combined an IRT model, a longitudinal model for underlying depression which describes the remission and then a possible relapse, and a hazard model for dropout depending on the evolution from baseline. The drug effect was best modelled as an effect on the remission and the relapse phases. The median predicted drop in HAMD between baseline and 6 weeks was 8.8 (90% PI, 8.3–9.2) when on placebo and 13.1 (90% PI, 12.8–13.4) when treated. Nine items were found to be the most informative.
Conclusion
The IRT framework allowed to characterise the evolution of depression with time and estimate the effect of agomelatine, as well as the link between symptoms and disease.






Similar content being viewed by others
References
WHO. World Health Organisation; 2018. Available from: https://www.who.int/en/news-room/fact-sheets/detail/depression. Accessed 13 Sept 2019.
Solomon DA, Keller MB, Leon AC, Mueller TI, Lavori PW, Shea MT, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157(2):229–33.
Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–38.
Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, et al. Cross-national epi-demiology of major depression and bipolar disorder. JAMA. 1996 Jul;276(4):293–9.
Van de Velde S, Bracke P, Levecque K. Gender differences in depression in 23 European countries. Cross-national variation in the gender gap in depression. Soc Sci Med. 2010 Jul;71:305–13.
Bentley SM, Pagalilauan GL, Simpson SA. Major depression. Med Clin N Am. 2014;98(5):981–1005.
De Bodinat C, Guardiola-Lemaitre B, Mocaër E, Renard P, Muñoz C, Millan MJ. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628.
Racagni G, Riva MA, Molteni R, Musazzi L, Calabrese F, Popoli M, et al. Mode of action of agomelatine: synergy between melatonergic and 5-HT2C receptors. World J Biol Psychiatry. 2011;12(8):574–87.
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–66.
Taylor D, Sparshatt A, Varma S, Olofinjana O. Antidepressant efficacy of agomelatine: meta-analysis of published and unpublished studies. Br Med J. 2014;348:g1888.
Khan A, Detke M, Khan SR, Mallinckrodt C. Placebo response and antidepressant clinical trial outcome. J Nerv Ment Dis. 2003;191(4):211–8.
Iovieno N, Papakostas GI. Correlation between different levels of placebo response rate and clinical trial outcome in major depressive disorder: a meta-analysis. J Clin Psychiatry. 2012;73(10):1300–6.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62.
Faries D, Herrera J, Rayamajhi J, DeBrota D, Demitrack M, Potter WZ. The responsiveness of the Hamilton depression rating scale. J Psychiatr Res. 2000;34(1):3–10.
Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton depression rating scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161(12):2163–77.
Bech P, Rafaelsen O. The use of rating scales exemplified by a comparison of the Hamilton and the Bech-Rafaelsen Melancholia Scale. Acta Psychiatr Scand. 1980;62(S285):128–32.
Gibbons RD, Clark DC, Kupfer DJ. Exactly what does the Hamilton depression rating scale measure? J Psychiatr Res. 1993;27(3):259–73.
Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–9.
Maier W, Philipp M. Improving the assessment of severity of depressive states: a reduction of the Hamilton Depression Scale. Pharmacopsychiatry. 1985;18(01):114–5.
McIntyre R, Kennedy S, Bagby RM, Bakish D. Assessing full remission. J Psychiatry Neurosci. 2002;27(4):235.
Ueckert S, Plan EL, Ito K, Karlsson MO, Corrigan B, Hooker AC, et al. Improved utilization of ADAS-cog assessment data through item response theory based pharmacometric modeling. Pharm Res. 2014;31(8):2152–65.
Buatois S, Retout S, Frey N, Ueckert S. Item response theory as an efficient tool to describe a heterogeneous clinical rating scale in de novo idiopathic Parkinson’s disease patients. Pharm Res. 2017;34(10):2109–18.
Bock RD. A brief history of item theory response. J Educ Meas. 1997;16(4):21–33.
Verma N, Beretvas SN, Pascual B, Masdeu JC, Markey MK. New scoring methodology improves the sensitivity of the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) in clinical trials. Alzheimers Res Ther. 2015;7(1):64.
Schneider LS, Kennedy RE, Wang G, Cutter GR. Differences in Alzheimer disease clinical trial outcomes based on age of the participants. Neurology. 2015;84(11):1121–7.
Dowling NM, Bolt DM, Deng S. An approach for estimating item sensitivity to within-person change over time: an illustration using the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog). Psychol Assess. 2016;28(12):1576.
Novakovic AM, Krekels EH, Munafo A, Ueckert S, Karlsson MO. Application of item response theory to modeling of expanded disability status scale in multiple sclerosis. AAPS J. 2017;19(1):172–9.
Vandemeulebroecke M, Bornkamp B, Krahnke T, Mielke J, Monsch A, Quarg P. A longitudinal item response theory model to characterize cognition over time in elderly subjects. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):635–41.
Välitalo P, Krekels E, van Dijk M, Simons S, Tibboel D, Knibbe C. Morphine pharmacodynamics in mechanically ventilated preterm neonates undergoing endotracheal suctioning. CPT Pharmacometrics Syst Pharmacol. 2017;6(4):239–48.
Gottipati G, Karlsson MO, Plan EL. Modeling a composite score in Parkinson’s disease using item response theory. AAPS J. 2017;19(3):837–45.
Krekels E, Novakovic AM, Vermeulen A, Friberg LE, Karlsson MO. Item response theory to quantify longitudinal placebo and paliperidone effects on PANSS scores in schizophrenia. CPT Pharmacometrics Syst Pharmacol. 2017;6(8):543–51.
Guk J, Chae D, Son H, Yoo J, Heo JH, Park K. Model-based assessment of the benefits and risks of recombinant tissue plasminogen activator treatment in acute ischaemic stroke. Br J Clin Pharmacol. 2018;84(11):2586–99.
Chae D, Park K. An item response theory based integrated model of headache, nausea, photophobia, and phonophobia in migraine patients. J Pharmacokinet Pharmacodyn. 2018;45(5):721–31.
Baker FB. The basics of item response theory. ERIC; 2001.
Gomeni R, Lavergne A, Merlo-Pich E. Modelling placebo response in depression trials using a longitu-dinal model with informative dropout. Eur J Pharm Sci. 2009;36(1):4–10.
Goodwin GM, Emsley R, Rembry S, Rouillon F. Agomelatine prevents relapse in patients with major depressive disorder without evidence of a discontinuation syndrome: a 24-week randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(8):1128–37.
Claghorn JL, Feighner JP. A double-blind comparison of paroxetine with imipramine in the long-term treatment of depression. J Clin Psychopharmacol. 1993;13:23S–7S.
Mitsikostas DD, Mantonakis L, Chalarakis N. Nocebo in clinical trials for depression: a meta-analysis. Psychiatry Res. 2014;215(1):82–6.
Mazumdar S, Tang G, Houck PR, Dew MA, Begley AE, Scott J, et al. Statistical analysis of longitudinal psychiatric data with dropouts. J Psychiatr Res. 2007;41(12):1032–41.
Little RJ. Modeling the drop-out mechanism in repeated-measures studies. J Am Stat Assoc. 1995;90(431):1112–21.
Hu C, Sale ME. A joint model for nonlinear longitudinal data with informative dropout. J Pharmacokinet Pharmacodyn. 2003;30(1):83–103.
Björnsson MA, Friberg LE, Simonsson US. Performance of nonlinear mixed effects models in the presence of informative dropout. AAPS J. 2015;17(1):245–55.
Pierre Olié J, Kasper S. Efficacy of agomelatine, a MT1/MT2 receptor agonist with 5-HT2C antagonistic properties, in major depressive disorder. Int J Neuropsychopharmacol. 2007;10(5):661–73.
Kennedy S, Emsley R. Placebo-controlled trial of agomelatine in the treatment of major depressive disorder. Eur Neuropsychopharmacol. 2006;16(2):93–100.
Kennedy SH, Avedisova A, Giménez-Montesinos N, Belaïdi C, agomelatine study group, et al. A placebo-controlled study of three agomelatine dose regimens (10 mg, 25 mg, 25-50 mg) in patients with major depressive disorder. Eur Neuropsychopharmacol. 2014;24(4):553–63.
Heun R, Ahokas A, Boyer P, Giménez-Montesinos N, Pontes-Soares F, Olivier V. The efficacy of agome-latine in elderly patients with recurrent major depressive disorder: a placebo-controlled study. J Clin Psychiatry. 2013;74(6):587–94.
Jacqmin P, Snoeck E, Van Schaick E, Gieschke R, Pillai P, Steimer JL, et al. Modelling response time profiles in the absence of drug concentrations: definition and performance evaluation of the K-PD model. J Pharmacokinet Pharmacodyn. 2007;34(1):57–85.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Erlbaum Associates; 1988.
Kjellsson MC, Zingmark PH, Jonsson EN, Karlsson MO. Comparison of proportional and differ-ential odds models for mixed-effects analysis of categorical data. J Pharmacokinet Pharmacodyn. 2008;35(5):483.
Ueckert S. Modeling composite assessment data using item response theory. CPT Pharmacometrics Syst Pharmacol. 2018;7(4):205–18.
Gomeni R, Merlo-Pich E. Bayesian modelling and ROC analysis to predict placebo responders using clinical score measured in the initial weeks of treatment in depression trials. Br J Clin Pharmacol. 2007;63(5):595–613.
Holford N, Li J, Benincosa L, Birath M. Population disease progress models for the time course of HAMD score in depressed patients receiving placebo in anti-depressant clinical trials. Abstracts of the XI annual meeting of the population approach group in Europe. 2002;Abstr. 311. Available from: www.page-meeting.org/?abstract=311. Accessed 13 Sept 2019.
Mould DR. Developing models of disease progression. In: Ette EI, Williams PJ, editors. Pharmacometrics: the science of quantitative pharmacology; 2007. p. 547–81.
Shang EY, Gibbs MA, Landen JW, Krams M, Russell T, Denman NG, et al. Evaluation of struc-tural models to describe the effect of placebo upon the time course of major depressive disorder. J Pharmacokinet Pharmacodyn. 2009;36(1):63–80.
Bauer JR, ICON S Development. NONMEM users guide: introduction to NONMEM 7.3.0. Maryland; 2013.
Russu A, Marostica E, De Nicolao G, Hooker AC, Poggesi I, Gomeni R, et al. Joint modeling of efficacy, dropout, and tolerability in flexible-dose trials: a case study in depression. Clin Pharmacol Ther. 2012;91(5):863–71.
Papp M, Gruca P, Boyer PA, Mocaër E. Effect of agomelatine in the chronic mild stress model of depression in the rat. Neuropsychopharmacology. 2003;28(4):694.
Singh SP, Singh V, Kar N. Efficacy of agomelatine in major depressive disorder: meta-analysis and appraisal. Int J Neuropsychopharmacol. 2012;15(3):417–28.
Koesters M, Guaiana G, Cipriani A, Becker T, Barbui C. Agomelatine efficacy and acceptability revisited: systematic review and meta-analysis of published and unpublished randomised trials. Br J Psychiatry. 2013;203(3):179–87.
Cusin C, Yang H, Yeung A, Fava M. Rating scales for depression. In: Handbook of clinical rating scales and assessment in psychiatry and mental health. Springer; 2009. p. 7–35.
Acknowledgements
The authors thank Valérie Olivier, Pierre-François Penelaud and Cécilia Gabriel Gracia for their clinical insight and challenging discussions. We would like to thank also Donato Teutonico and Karl Brendel for their valuable contribution to this work as well as Hervé Le Nagard and Lionel de la Tribouille for the use of the computer cluster services hosted on the “Centre de Biomodélisation UMR1137”. This work is also indebted to the investigators in the Goodwin et al. (36), Olie, Kennedy and Emsley (44), Kennedy et al. (45) and Heun (46) studies.
Funding
Marc Cerou received funding from Institut de Recherches Internationales Servier, as part of a PhD research fellowship programme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Emmanuelle Comets and Marylore Chenel are co-last authors
Electronic Supplementary Material
ESM 1
(PDF 2273 kb)
Rights and permissions
About this article
Cite this article
Cerou, M., Peigné, S., Comets, E. et al. Application of Item Response Theory to Model Disease Progression and Agomelatine Effect in Patients with Major Depressive Disorder. AAPS J 22, 4 (2020). https://doi.org/10.1208/s12248-019-0379-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0379-x