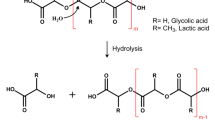
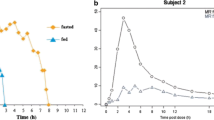
Abstract
Drug product specifications are a critical element of a good control strategy. Parenteral microsphere products are complex dosage forms, requiring careful development of test methods and acceptance criteria for the specifications. In particular, the in vitro release test method and acceptance criteria require rigorous scientific consideration and should be developed with an eye toward understanding the mechanisms of drug release. The final specifications need to ensure the safety, identity, strength, performance, and quality of the drug product at release and during storage through the end of its shelf-life. The specification limits are typically established based upon regulatory guidance, available data from the manufacturing process (process capability), from non-clinical, clinical, and stability studies.





Similar content being viewed by others
References
Jalil R, Nixon JR. Biodegradable poly (lactic acid) and poly (lactide-co-glycolide) microcapsules: problems associated with preparative techniques and release properties. J Microencapsulation. 1990;7(3):297–325.
Tamilvanan S, Babu VR, Kannan K, Basu SK, Sa B. Manufacturing techniques and excipients used during the design of biodegradable polymer-based microspheres containing therapeutic peptide/protein for parenteral controlled drug delivery. PDA J Pharm Sci Technol. 2008;62(2):125–54.
D'Souza SS, DeLuca PP. Methods to Assess in vitro drug release from injectable polymeric particulate systems. Pharm Res. 2006;23(3):460–74.
http://www.vivitrol.com accessed May 14, 2009.
Q6A International Conference on Harmonisation; Guidance on Q6A Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances. 2000 May. http://www.emea.europa.eu/pdfs/human/ich/036796en.pdf. accessed May 14, 2009.
Martinez M, Rathbone M, Burgess D, Huynh M. In vitro and in vivo considerations associated with parenteral sustained release products: a review based upon information presented and points expressed at the 2007 Controlled Release Society Annual Meeting. J Control Release. 2008;129(2):79–87.
FDA Guidance for Industry. Container and closure system integrity testing in lieu of sterility testing as a component of the stability protocol for sterile products. 2008 Feb. http://www.fda.gov/cber/gdlns/contain.htm accessed May 14, 2009.
Akers MJ, Fites AL, Robison RL. Formulation design and development of parenteral suspensions. J Parent Sci Technol. 1987;41(3):88–96.
Reich G. Ultrasound-induced degradation of PLA and PLGA during microsphere processing: influence of formulation variables. Eur J Pharm Biopharm. 1998;45:165–71.
Cha Y, Pitt CG. The acceleration of degradation-controlled drug delivery from polyester microspheres. J Control Release. 1989;8(3):259–65.
The United States Pharmacopoeia (USP), 31st Edition (NF 26); 2008 Dec.
Toguchi H. Sterility assurance of microspheres. J Control Release. 1999;62(1–2):51–5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Marilyn Martinez and Michael Rathbone
Rights and permissions
About this article
Cite this article
Kumar, R., Palmieri, M.J. Points to Consider when Establishing Drug Product Specifications for Parenteral Microspheres. AAPS J 12, 27–32 (2010). https://doi.org/10.1208/s12248-009-9156-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-009-9156-6