Abstract
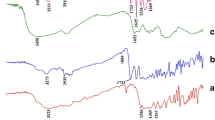
The main objective of this study was to develop a local, oral mucoadhesive metronidazole benzoate (MET) delivery system that can be applied and removed by the patient for the treatment of periodontal diseases. Mucoadhesive micromatricial chitosan/poly(ε-caprolactone) (CH/PCL) films and chitosan films were prepared. thermal behavior, morphology, and particle size measurements were used to evaluate the prepared films. The effect of different molar masses of CH and different ratios of medium Mwt molar mass chitosan (MCH):PCL on water absorption, in vitro bioadhesion, mechanical properties, and in vitro drug release was examined. In vivo performance of the selected formulation was also evaluated. Differential scanning calorimetry examination revealed that MET existed mainly in amorphous form. Under microscopic examination, PCL microparticles were homogeneously dispersed in the films. The use of different molar masses of CH and different ratios of (MCH):PCL affected the size of the entrapped particles. Addition of PCL significantly decreased percentage water uptake and bioadhesion force compared with pure CH film. With regard to mechanical properties, the 2-layered film containing 1∶0.625 MCH:PCL had the best tensile properties. At fixed CH:PCL ratio (1∶1.25), the slowest drug release was obtained from films containing high molar mass CH. On the other hand, the 2-layered film that consisted of 1∶0.625 MCH:PCL had the slowest MET release. In vivo evaluation of the selected film revealed that metronidazole concentration in saliva over 6 hours ranged from 5 to 15 μg/mL, which was within and higher than the reported range of minimum inhibitory concentration for metronidazole. A significant in vitro/in vivo correlation under the adopted experimental conditions was obtained.
Similar content being viewed by others
References
Piovano S. Bacteriology of most frequent oral anaerobic infections.Anaerobe. 1999;5:221–227.
Perioli L, Ambrogi V, Rubini D, et al. Novel mucoadhesive buccal formulation containing metronidazole for the treatment of periodontal disease.J Control Release. 2004;95:521–533.
Schwach-Abdellaoui K, Vivien-Castioni N, Gurny R. Local delivery of antimicrobial agents for the treatment of periodontal diseases.Eur J Pharm Biopharm. 2000;50:83–99.
Vyas SP, Sihorkar V, Mishra V. Controlled and targeted drug delivery strategies towards intraperiodontal pocket diseases.J Clin Pharm Ther. 2000;25:21–42.
Brackett MG, Drisko CL, Thompson AL, Waller JL, Marshall DL, Schuster GS. Penetration of fluids into periodontal pockets using a powered toothbrush/irrigator device.J Contemp Dent Pract. 2006; 7:30–39.
Ahuja A, Ali J, Rahman S. Biodegradable periodontal intrapocket device containing metronidazole and amoxycillin: formulation and characterisation.Pharmazie. 2006;61:25–29.
Barat R, Srinatha A, Pandit JK, et al. Niridazole biodegradable inserts for local long-term treatment of periodontitis: possible new life for an orphan drug.Drug Deliv. 2006;13:365–373.
Samati Y, Yuksel N, Tarimci N. Preparation and characterization of poly (D L-lactic-co-glycolic acid) microspheres containing flurbiprofen sodium.Drug Deliv. 2006;13:105–111.
Liebana J, Castillo AM, Alvarez M. Periodontal diseases: microbiological considerations.Med Oral Patol Oral Cir Bucal. 2004;9:82–91.
Zheng LY, Zhu JF. Study on antimicrobial activity of chitosan with different molecular weights.Carbohydr Polym. 2003;54:527–530.
Perugini P, Genta I, Conti B, Modena T, Pavanetto F. Periodontal delivery of ipriflavone: new chitosan/PLGA film delivery system for a lipophilic drug.Int J Pharm. 2003;252:1–9.
USP.US Pharmacopeia National Formulary. vol. 21. Rockville, MD: USP; 1999.
Patty PJ, Frisken BJ. Direct determination of the number-weighted mean radius and polydispersity from dynamic light-scattering data.Appl Opt. 2006;45:2209–2216.
Peh KK, Wong CF. Polymeric films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties.J Pharm Pharm Sci. 1999;2:53–61.
Baro M, Sanchez E, Delgado A, Perera A, Evora C. In vitro-in vivo characterization of gentamicin bone implants.J Control Release. 2002;83:353–364.
Ponchel G, Touchard F, Duchene D, Peppas NA. Bioadhesive analysis of controlled-release systems, I: fracture and interpenetration analysis in poly(acrylic acid)-containing systems.J Control Release. 1987;5:129–141.
Wong CF, Yuen KH, Peh KK. Formulation and evaluation of controlled release Eudragit buccal patches.Int J Pharm. 1999; 178:11–22.
Padula CP, Colombo G, Nicoli S, Catellani PL, Massimo G, Santi P. Bioadhesive film for the transdermal delivery of lidocaine: in vitro and in vivo behavior.J Control Release. 2003;88:277–285.
Ritger PL, Peppas NA. A simple equation for description of solute release, II: Fickian and anomalous release from swellable devices.J Control Release. 1987;5:37–42.
Metz P, Kohlhepp SJ, Gilbert DN. Study of different off-line sample processing procedures and the measurement of antibiotic and antiviral levels in human serum by high-performance liquid chromatography.J Chromatogr B. 2002;773:159–166.
Sarasam A, Madihally SV. Characterization of chitosanpolycaprolactone blends for tissue engineering applications.Biomaterials. 2005;26:5500–5508.
Roldo M, Hornof M, Caliceti P, Bernkop-Schnurch A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: synthesis and in vitro evaluation.Eur J Pharm Biopharm. 2004;57:115–121.
Wenling C, Duohui J, Jiamou L, Yandao G, Nanming Z, Xiufang Z. Effects of the degree of deacetylation on the physicochemical properties and Schwann cell affinity of chitosan films.J Biomater Appl. 2005; 20:157–177.
Needleman IG, Smales FC. In vitro assessment of bioadhesion for periodontal and buccal drug delivery.Biomaterials. 1995;16:617–624.
Harding SE. Trends in mucoadhesive analysis.Trends Food Sci Tech. 2006;17:255–262.
Ikinci G, Senel S, Akincibay H, et al. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis.Int J Pharm. 2002; 235:121–127.
Dhanikula AB, Panchagnula R. Development and characterization of biodegradable chitosan films for local delivery of paclitaxel.AAPS J. 2004;6: Article 27.
Martin AN, ed. Polymer science. In:Physical Pharmacy: Physical Chemical Principles in the Pharmaceutical Sciences. 4th ed. Philadelphia, PA Lea & Febiger; 1993:575–578.
Lorenzo-Lamosa ML, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Design of microencapsulated chitosan microspheres for colonic drug delivery.J Control Release. 1998;52:109–118.
Sarasam AR, Krishnaswamy RK, Madihally SV. Blending chitosan with polyoaprolactone: effects on physicochemical and antibacterial properties.Biomacromolecules. 2006;7:1131–1138.
Colombo P, Santi P, Bettini R, Brazel CS. Drug release from swelling controlled systems. In: Wise DL, ed.Handbook of Pharmaceutical Controlled Release Technology. vol. 9. New York, NY: Marcel Dekker; 2000:183–209.
Khanna R, Agarwal SP, Ahuja A. Mucoadhesive buccal tablets of cłotimazole for oral candidiasis.Drug Dev Ind Pharm. 1997; 23:1–7.
Mody SSB, Mody PD, Doshi MM, inventors. Pharmaceutical dental formulation for topical application of metronidazole benzoate and chlorhexidine gluconate. US patent 6 017 516. January 25, 2000.
Parfitt K.Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press; 1999.
Milazzo I, Blandino G, Musumeci R, Nicoletti G, Lo Bue AM, Speciale A. Antibacterial activity of moxifloxacin against periodontal anaerobic pathogens involved in systemic infections.Int J Antimicrob Agent. 2002;20:451–456.
Mckellar QA, Sanchez Bruni SF, Jones DG. Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine.J Vet Pharmacol Ther. 2004;27:503–514.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: September 14, 2007
Rights and permissions
About this article
Cite this article
El-Kamel, A.H., Ashri, L.Y. & Alsarra, I.A. Micromatricial metronidazole benzoate film as a local mucoadhesive delivery system for treatment of periodontal diseases. AAPS PharmSciTech 8, 75 (2007). https://doi.org/10.1208/pt0803075
Received:
Revised:
Accepted:
DOI: https://doi.org/10.1208/pt0803075