Abstract
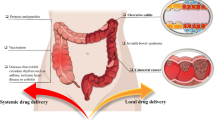
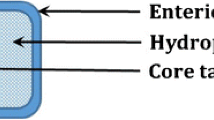
The human race is consistently striving for achieving good health and eliminate disease-causing factors. For the last few decades, scientists have been endeavoring to invent and innovate technologies that can substitute the conventional dosage forms and enable targeted and prolonged drug release at a particular site. The novel multi-matrix technology is a type of matrix formulation where the formulation is embraced to have a matrix system with multiple number of matrices. The MMX technology embraces with a combination of outer hydrophilic layer and amphiphilic/lipophilic core layer, within which drug is encapsulated followed by enteric coating for extended/targeted release at the required site. In comparison to conventional oral drug delivery systems and other drug delivery systems, multi-matrix (MMX) technology formulations afford many advantages. Additionally, it attributes for targeting strategy aimed at the colon and offers modified prolonged drug release. Thus, it has emerged rapidly as a potential alternative option in targeted oral drug delivery. However, the development of this MMX technology formulations is a exigent task and also has its own set of limitations. Due to its promising advantages and colon targeting strategy over the other colon targeted drug delivery systems, premier global companies are exploiting its potential. This article review deep insights into the formulation procedures, drug delivery mechanism, advantages, limitations, safety and efficacy studies of various marketed drug formulations of MMX technology including regulatory perspectives and future perspectives.








Similar content being viewed by others
References
Sastry SV, Nyshadham JR, Fix JA. Recent technological advances in oral drug delivery–a review. Pharm Sci Technol Today. 2000;3(4):138–45.
Varma MV, et al. Factors affecting mechanism and kinetics of drug release from matrix-based oral controlled drug delivery systems. Am J Drug Deliv. 2004;2(1):43–57.
Tiwari SB, Rajabi-Siahboomi AR. Extended-release oral drug delivery technologies: monolithic matrix systems. Drug delivery systems. 2008;437:217–43.
Kotla N, Shivapooja A. Recent developments in colon specific drug delivery systems approaches promising in targeting colon. Int J Pharm Clin Res. 2014;6:101–6.
Grobman B. MULTIPLE MYELOMA PRESENTING AS CVA. Minerva Med. 1982;73(34):2183–8.
Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019;12(2):113.
Xavier R, Podolsky D. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–34.
Bayan MF, Bayan RF. Recent advances in mesalamine colonic delivery systems. Future Journal of Pharmaceutical Sciences. 2020;6(1):1–7.
Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. The Lancet. 2007;369(9573):1641–57.
Christophi GP, Rengarajan A, Ciorba MA. Rectal budesonide and mesalamine formulations in active ulcerative proctosigmoiditis: efficacy, tolerance, and treatment approach. Clin Exp Gastroenterol. 2016;9:125.
Thia KT, et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139(4):1147–55.
Le Berre C, et al. Ulcerative colitis and Crohn’s disease have similar burden and goals for treatment. Clin Gastroenterol Hepatol. 2020;18(1):14–23.
Nardelli S, et al. MMX® technology and its applications in gastrointestinal diseases. Ther Adv Gastroenterol. 2017;10(7):545–52.
Murray A, et al. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews. 2020;8(8):CD000543.
Liu P, et al. Receptor-mediated targeted drug delivery systems for treatment of inflammatory bowel disease: opportunities and emerging strategies. Acta Pharmaceutica Sinica B, 2020.
Tindall WN. New approaches to adherence issues when dosing oral aminosalicylates in ulcerative colitis. Am J Health Syst Pharm. 2009;66(5):451–7.
Khan S, et al. Real-world evidence on adherence, persistence, switching and dose escalation with biologics in adult inflammatory bowel disease in the United States: a systematic review. J Clin Pharm Ther. 2019;44(4):495–507.
Kotla NG, et al. Bioresponsive drug delivery systems in intestinal inflammation: State-of-the-art and future perspectives. Adv Drug Deliv Rev. 2019;146:248–66.
Prantera C, et al. A new oral delivery system for 5-ASA: preliminary clinical findings for MMx. Inflamm Bowel Dis. 2005;11(5):421–7.
Kuenzig ME, et al. Budesonide for maintenance of remission in Crohn’s disease. Cochrane Database of Systematic Reviews. 2014;2014(8):CD002913.
Salice M, et al. A current overview of corticosteroid use in active ulcerative colitis. Expert Rev Gastroenterol Hepatol. 2019;13(6):557–61.
Harbord M, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohn’s Colitis. 2017;11(7):769–84.
Mantzaris GJ, et al. How adherent to treatment with azathioprine are patients with Crohn’s disease in long-term remission? Inflamm Bowel Dis. 2007;13(4):446–50.
Mottet C, et al. Experts opinion on the practical use of azathioprine and 6-mercaptopurine in inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(11):2733–47.
Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am J Gastroenterol. 2010;105(3):501–23.
Nanda K, Moss AC. Update on the management of ulcerative colitis: treatment and maintenance approaches focused on MMX® mesalamine. Clinical pharmacology: advances and applications. 2012;4:41.
Qian X, et al. Low dose of azathioprine is effective to induce and maintain remission in active Crohn disease: A prospective observational study. Medicine. 2018;97(34):e11814.
Pasternak B, et al. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. 2013;177(11):1296–305.
Armstrong RG, West J, Card TR. Risk of cancer in inflammatory bowel disease treated with azathioprine: a UK population-based case–control study. Am J Gastroenterol. 2010;105(7):1604–9.
de Boer NK, et al. Thiopurines in inflammatory bowel disease: new findings and perspectives. J Crohns Colitis. 2018;12(5):610–20.
Oliva-Hemker MM, et al. Nonadherence with thiopurine immunomodulator and mesalamine medications in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2007;44(2):180–4.
Pogoda K, et al. Effects of BRCA Germline Mutations on Triple-Negative Breast Cancer Prognosis. J Oncol. 2020;2020:8545643.
Colombel JF, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–95.
Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res. 2018;11:215.
Rutgeerts P, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–76.
Moss AC, Peppercorn MA. Steroid-refractory severe ulcerative colitis. Drugs. 2008;68(9):1157–67.
Kamm MA, et al. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology. 2007;132(1):66–75.
Fiorino G, et al. New drug delivery systems in inflammatory bowel disease: MMX™ and tailored delivery to the gut. Curr Med Chem. 2010;17(17):1851–7.
Belali N, Wathoni N, Muchtaridi M. Advances in orally targeted drug delivery to colon. Journal of advanced pharmaceutical technology & research. 2019;10(3):100.
Koutroubakis IE. Recent advances in the management of distal ulcerative colitis. World journal of gastrointestinal pharmacology and therapeutics. 2010;1(2):43.
Phad AB, et al. Matrix Tablet: As A Sustained Release Drug Delivery System. World Journal of Pharmaceutical Research. 2014;3(5):1377–1390.
Fernandez-Becker NQ, Moss AC. Improving delivery of aminosalicylates in ulcerative colitis. Drugs. 2008;68(8):1089–103.
Moro et al. United States Patent, KR101763700B1, Solid composition for the oral administration of dyes and diagnostic use thereof. korea.
Joshi M. Role of eudragit in targeted drug delivery. Int J Curr Pharm Res. 2013;5(2):58–62.
Ford JL, Rubinstein MH, Hogan JE. Formulation of sustained release promethazine hydrochloride tablets using hydroxypropyl-methylcellulose matrices. Int J Pharm. 1985;24(2–3):327–38.
Bettini R, et al. Translocation of drug particles in HPMC matrix gel layer: effect of drug solubility and influence on release rate. J Control Release. 2001;70(3):383–91.
Ghori MU, Conway BR. Hydrophilic matrices for oral control drug delivery. Am J Pharmacol Sci. 2015;3(5):103–9.
Rao KR, Devi KP, Buri P. Influence of molecular size and water solubility of the solute on its release from swelling and erosion controlled polymeric matrices. J Control Release. 1990;12(2):133–41.
Nautyal U, Gupta D. Oral Sustained Release Tablets: An Overview With A Special Emphasis On Matrix Tablet. International Journal of Health and Biological Sciences. 2020;3(1):06–13.
Sujja-Areevath J, et al. Relationship between swelling, erosion and drug release in hydrophillic natural gum mini-matrix formulations. Eur J Pharm Sci. 1998;6(3):207–17.
Maggi L, et al. Dissolution behaviour of hydrophilic matrix tablets containing two different polyethylene oxides (PEOs) for the controlled release of a water-soluble drug. Dimensionality study Biomaterials. 2002;23(4):1113–9.
Tahara K, Yamamoto K, Nishihata T. Overall mechanism behind matrix sustained release (SR) tablets prepared with hydroxypropyl methylcellulose 2910. J Control Release. 1995;35(1):59–66.
Nokhodchi A, et al. The role of oral controlled release matrix tablets in drug delivery systems. BioImpacts. 2012;2(4):175.
Cellulose, M.a.W.M. MethocelTM and WellenceTM Modified Cellulose. Nutrition & Biosciences, 2020. www.dupontnutritionandbiosciences.com
Levina M. Application of a modelling system in the formulation of extended release hydrophilic matrices. Pharmaceutical Technology Europe. 2006;18(7):20–6.
Ebube NK, Jones AB. Sustained release of acetaminophen from a heterogeneous mixture of two hydrophilic non-ionic cellulose ether polymers. Int J Pharm. 2004;272(1–2):19–27.
Ghori MU, et al. Simultaneous quantification of drug release and erosion from hypromellose hydrophilic matrices. Int J Pharm. 2014;465(1–2):405–12.
Klančar U, et al. Determining the polymer threshold amount for achieving robust drug release from HPMC and HPC matrix tablets containing a high-dose BCS class I model drug: in vitro and in vivo studies. AAPS PharmSciTech. 2015;16(2):398–406.
Jain AK, et al. The influence of hydroxypropyl methylcellulose (HPMC) molecular weight, concentration and effect of food on in vivo erosion behavior of HPMC matrix tablets. J Control Release. 2014;187:50–8.
Asare-Addo K, et al. The influence of agitation sequence and ionic strength on in vitro drug release from hypromellose (E4M and K4M) ER matrices—The use of the USP III apparatus. Colloids Surf, B. 2013;104:54–60.
Viridén A, Larsson A, Wittgren B. The effect of substitution pattern of HPMC on polymer release from matrix tablets. Int J Pharm. 2010;389(1–2):147–56.
Escudero J, Ferrero C, Jiménez-Castellanos M. Compaction properties, drug release kinetics and fronts movement studies from matrices combining mixtures of swellable and inert polymers: Effect of HPMC of different viscosity grades. Int J Pharm. 2008;351(1–2):61–73.
Escudero J, Ferrero C, Jiménez-Castellanos M. Compaction properties, drug release kinetics and fronts movement studies from matrices combining mixtures of swellable and inert polymers. II. Effect of HPMC with different degrees of methoxy/hydroxypropyl substitution. Int J Pharmac. 2010;387(1–2):56–64.
Shah NH, et al. Effect of processing techniques in controlling the release rate and mechanical strength of hydroxypropyl methylcellulose based hydrogel matrices. Eur J Pharm Biopharm. 1996;42(3):183–7.
Patel H, et al. Matrix type drug delivery system: A review. J Pharm Sci Biosci Res. 2011;1(3):143–51.
Mamidala RK, et al. Factors influencing the design and performance of oral sustained/controlled release dosage forms. Int J Pharm Sci and Nanotech. 2009;2(3):583–94.
Anita, Singh A, Dabral A. A review on colon targeted drug delivery system. Int J Pharmaceut Sci Res. 2019;10(1):47–56.
Villa Roberto et al. "Mesalazine controlled release oral pharmaceutical compositions." U.S. Patent No. 6,773,720. 10 Aug. 2004.
Mauro Ajani, L.M.I.L.M., Lainate (Milano) (IT): Roberto Villa, Lainate (Milano) (IT), Pharmaceutical Compositions for the Oral Administration of Heparn or Dervatives thereof, US 9,308,220 B2. 2016, Cosmo Technologies Limited, Wicklow (IE).
Conti S, et al. Matrices containing NaCMC and HPMC: 1. Dissolution performance characterization. Int J Pharmaceut. 2007;333(1–2):136–42.
Conti S, et al. Matrices containing NaCMC and HPMC: 2 Swelling and release mechanism study. Int J pharmaceut. 2007;333(1–2):143–51.
Maderuelo C, Zarzuelo A, Lanao JM. Critical factors in the release of drugs from sustained release hydrophilic matrices. J Control Release. 2011;154(1):2–19.
Malamataris S, Karidas T. Effect of particle size and sorbed moisture on the tensile strength of some tableted hydroxypropyl methylcellulose (HPMC) polymers. Int J Pharm. 1994;104(2):115–23.
Omidian H, Park K. Swelling agents and devices in oral drug delivery. Journal of drug delivery science and technology. 2008;18(2):83–93.
Ferrero C, Massuelle D, Doelker E. Towards elucidation of the drug release mechanism from compressed hydrophilic matrices made of cellulose ethers. II. Evaluation of a possible swelling-controlled drug release mechanism using dimensionless analysis. J Control Release. 2010;141(2):223–33.
Gao P, et al. Swelling of hydroxypropyl methylcellulose matrix tablets. 2. Mechanistic study of the influence of formulation variables on matrix performance and drug release. J Pharmaceut Sci. 1996;85(7):732–40.
Enayatifard R, et al. Effect of hydroxypropyl methylcellulose and ethyl cellulose content on release profile and kinetics of diltiazem HCl from matrices. Tropical Journal of Pharmaceutical Research. 2009;8(5):48086.
Lucinda-Silva RM, Salgado HRN, Evangelista RC. Alginate–chitosan systems: in vitro controlled release of triamcinolone and in vivo gastrointestinal transit. Carbohyd Polym. 2010;81(2):260–8.
Amidon S, Brown JE, Dave VS. Colon-targeted oral drug delivery systems: design trends and approaches. AAPS PharmSciTech. 2015;16(4):731–41.
Sandborn W, et al. MMX Multi Matrix System® mesalazine for the induction of remission in patients with mild-to-moderate ulcerative colitis: a combined analysis of two randomized, double-blind, placebo-controlled trials. Aliment Pharmacol Ther. 2007;26(2):205–15.
Campbell D, Pethrick RA, White JR. Polymer Characterization Physical Techniques. Chapman and Hall. Polymer characterization - Wikipedia. 1989; pp 11–13
Su W-F. Characterization of Polymer. In: Principles of Polymer Design and Synthesis. Berlin: Springer; 2013. p. 89–110.
Van Lieshout MH, et al. Characterization of polymers by multi-step thermal desorption/programmed pyrolysis gas chromatography using a high temperature PTV injector. J High Resolut Chromatogr. 1996;19(4):193–9.
Wang L, et al. Design and evaluation of hydrophilic matrix system containing polyethylene oxides for the zero-order controlled delivery of water-insoluble drugs. AAPS PharmSciTech. 2017;18(1):82–92.
Körner A, et al. Influence of different polymer types on the overall release mechanism in hydrophilic matrix tablets. Molecules. 2009;14(8):2699–716.
Arai K, Shikata T. Hydration/Dehydration Behavior of Hydroxyethyl Cellulose Ether in Aqueous Solution. Molecules. 2020;25(20):4726.
Zahoor FD, et al. Investigation of within-tablet dynamics for extended release of a poorly soluble basic drug from hydrophilic matrix tablets using ATR–FTIR imaging. Mol Pharm. 2020;17(4):1090–9.
Quodbach J, Kleinebudde P. Performance of tablet disintegrants: impact of storage conditions and relative tablet density. Pharm Dev Technol. 2015;20(6):762–8.
Chen YY, et al. Quantitative ultra-fast MRI of HPMC swelling and dissolution. J Pharm Sci. 2010;99(8):3462–72.
Chen C, Gladden LF, Mantle MD. Direct visualization of in vitro drug mobilization from Lescol XL tablets using two-dimensional 19F and 1H magnetic resonance imaging. Mol Pharm. 2014;11(2):630–7.
Tajarobi F, et al. Simultaneous probing of swelling, erosion and dissolution by NMR-microimaging—effect of solubility of additives on HPMC matrix tablets. Eur J Pharm Sci. 2009;37(2):89–97.
Nott KP. Magnetic resonance imaging of tablet dissolution. Eur J Pharm Biopharm. 2010;74(1):78–83.
Yassin S, et al. Diffusion and swelling measurements in pharmaceutical powder compacts using terahertz pulsed imaging. J Pharm Sci. 2015;104(5):1658–67.
Mašková E, et al. Highly soluble drugs directly granulated by water dispersions of insoluble eudragit® polymers as a part of hypromellose K100M matrix systems. BioMed research international. 2019;2019:8043415.
Hiremath P, Nuguru K, Agrahari V. Material attributes and their impact on wet granulation process performance. In: Handbook of pharmaceutical wet granulation. Elsevier; 2019. p. 263–315.
Prakash K, Reddy B, Sreenivasulu V. Effect of tablet surface area and surface area/volume on drug release from lamivudine extended release matrix tablets. International Journal of Pharmaceutical Sciences and Nanotechnology. 2010;3(1):872–6.
Mamani PL, Ruiz-Caro R, Veiga MD. Matrix tablets: the effect of hydroxypropyl methylcellulose/anhydrous dibasic calcium phosphate ratio on the release rate of a water-soluble drug through the gastrointestinal tract I. vitro tests. Aaps Pharmscitech. 2012;13(4):1073–83.
Prasanth V, Jayaprakash R, Mathew ST. Colon specific drug delivery systems: a review on various pharmaceutical approaches. J Appl Pharm Sci. 2012;2(01):163–9.
Zarmpi P, et al. Biopharmaceutical aspects and implications of excipient variability in drug product performance. Eur J Pharm Biopharm. 2017;111:1–15.
Akbari J, et al. Influence of hydroxypropyl methylcellulose molecular weight grade on water uptake, erosion and drug release properties of diclofenac sodium matrix tablets. Trop J Pharm Res. 2011;10(5):535–41.
Tritt-Goc J, Kowalczuk J, Pislewski N. Hydration of hydroxypropylmethyl cellulose: Effects of pH and molecular mass. Acta Physica Polonica-Series A General Physics. 2005;108(1):197–206.
Greiderer A, et al. Characterization of hydroxypropylmethylcellulose (HPMC) using comprehensive two-dimensional liquid chromatography. J Chromatogr A. 2011;1218(34):5787–93.
Kavanagh N, Corrigan OI. Swelling and erosion properties of hydroxypropylmethylcellulose (Hypromellose) matrices—influence of agitation rate and dissolution medium composition. Int J Pharm. 2004;279(1–2):141–52.
Pajander J, et al. Behaviour of HPMC compacts investigated using UV-imaging. Int J Pharm. 2012;427(2):345–53.
Ju RT, Nixon PR, Patel MV. Drug release from hydrophilic matrices. 1. New scaling laws for predicting polymer and drug release based on the polymer disentanglement concentration and the diffusion layer. Journal of pharmaceutical sciences. 1995;84(12):1455–63.
Reynolds TD, et al. Polymer erosion and drug release characterization of hydroxypropyl hethylcellulose matrices. J Pharm Sci. 1998;87(9):1115–23.
Brady J, et al. Polymer properties and characterization. In: Developing solid oral dosage forms. Elsevier; 2017. p. 181–223.
Mastropietro DJ, Park K, and Omidian H. Polymers in oral drug delivery. 2017. Faculty Books and Book Chapters, edition 2, chapter 23. https://nsuworks.nova.edu/hpd_corx_facbooks/17
Gustafsson C, et al. Characterisation of particle properties and compaction behaviour of hydroxypropyl methylcellulose with different degrees of methoxy/hydroxypropyl substitution. Eur J Pharm Sci. 1999;9(2):171–84.
Holte Ø, et al. Sustained release of water-soluble drug from directly compressed alginate tablets. Eur J Pharm Sci. 2003;20(4–5):403–7.
Liew CV, et al. Evaluation of sodium alginate as drug release modifier in matrix tablets. Int J Pharm. 2006;309(1–2):25–37.
Nish S, Mathew G, Lincy J. Matrix tablets: an effective way for oral controlled release drug delivery. Iran J Pharmaceut Sci. 2012;8(3):165–70.
Medicines, E.D.f.t.Q.o., European pharmacopoeia. 2007: Council of Europe.
Matero S, et al. Predicting the drug concentration in starch acetate matrix tablets from ATR-FTIR spectra using multi-way methods. Anal Chim Acta. 2007;595(1–2):190–7.
Khaled SA, et al. 3D printing of tablets containing multiple drugs with defined release profiles. Int J Pharm. 2015;494(2):643–50.
Krishnaiah Y, Karthikeyan R, Satyanarayana V. A three-layer guar gum matrix tablet for oral controlled delivery of highly soluble metoprolol tartrate. Int J Pharm. 2002;241(2):353–66.
Marinich J, Ferrero C, Jiménez-Castellanos M. Graft copolymers of ethyl methacrylate on waxy maize starch derivatives as novel excipients for matrix tablets: Physicochemical and technological characterisation. Eur J Pharm Biopharm. 2009;72(1):138–47.
Muñoz-Ruiz A, Jiménez-Castellanos M. Integrated system of data acquisition for measure of flow rate. Pharm Technol Int Biopharm. 1993;8:21–9.
Heckel R. Density-pressure relationships in powder compaction. Trans Metall Soc AIME. 1961;221(4):671–5.
Heckel W. An analysis of powder compaction phenomena. Trans Metall Soc AIME. 1961;221:671–5.
Moes JJ, et al. Application of process analytical technology in tablet process development using NIR spectroscopy: Blend uniformity, content uniformity and coating thickness measurements. Int J Pharm. 2008;357(1–2):108–18.
Baumgartner S, et al. Quantitative evaluation of polymer concentration profile during swelling of hydrophilic matrix tablets using 1H NMR and MRI methods. Eur J Pharm Biopharm. 2005;59(2):299–306.
Hyde T, Gladden L, Payne R. A nuclear magnetic resonance imaging study of the effect of incorporating a macromolecular drug in poly (glycolic acid-co-DL-lactic acid). J Control Release. 1995;36(3):261–75.
Tritt-Goc J, Piślewski N. Magnetic resonance imaging study of the swelling kinetics of hydroxypropylmethylcellulose (HPMC) in water. J Control Release. 2002;80(1–3):79–86.
Guo H, Heinämäki J, Yliruusi J. Characterization of particle deformation during compression measured by confocal laser scanning microscopy. Int J Pharm. 1999;186(2):99–108.
Adler J, Jayan A, Melia CD. A method for quantifying differential expansion within hydrating hydrophilic matrixes by tracking embedded fluorescent microspheres. J Pharm Sci. 1999;88(3):371–7.
Pygall SR, et al. Pharmaceutical applications of confocal laser scanning microscopy: The physical characterisation of pharmaceutical systems. Adv Drug Deliv Rev. 2007;59(14):1434–52.
Cutts L, et al. Solute and water transport within the gel layer of hydrating HPMC tablets. In: Proc Int Symp Control Release Bioact Mater. 1995.
Brunner M, et al. Gastrointestinal transit and 5-ASA release from a new mesalazine extended-release formulation. Aliment Pharmacol Ther. 2003;17(3):395–402.
Williams C, et al. Optimizing clinical use of mesalazine (5-aminosalicylic acid) in inflammatory bowel disease. Ther Adv Gastroenterol. 2011;4(4):237–48.
Qureshi AI, Cohen RD. Mesalamine delivery systems: do they really make much difference? Adv Drug Deliv Rev. 2005;57(2):281–302.
Cohen R. evolutionary advances in the delivery of aminosalicylates for the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2006;24(3):465–74.
Pharmaceuticals C. LIALDATM MMX treatment for ulcerative colitis gets FDA approval, https://www.cosmopharma.com/. 2007 [cited 2007; Available from: https://www.cosmopharma.com/.
Iacucci M, de Silva S, Ghosh S. Mesalazine in inflammatory bowel disease: a trendy topic once again? Can J Gastroenterol. 2010;24(2):127–33.
McCormack PL, Robinson DM, Perry CM. Delayed-release Multi Matrix System (MMX [TM]) mesalazine: in ulcerative colitis. Drugs. 2007;67(17):2635–43.
Kedia P, Cohen RD. Once-daily MMX mesalamine for the treatment of mild-to-moderate ulcerative colitis. Ther Clin Risk Manag. 2007;3(5):919.
Sandborn WJ, et al. Once-daily dosing of delayed-release oral mesalamine (400-mg tablet) is as effective as twice-daily dosing for maintenance of remission of ulcerative colitis. Gastroenterology. 2010;138(4):1286-1296.e3.
Hinojosa J, Navas V, Saro C. Pharmacokinetics: efficacy and safety of MMX mesalamine formulation for treating ulcerative colitis. Revista Colombiana de Gastroenterologia. 2014;29(1):46–54.
Roberto Villa, L.I.M.P., Cignese (IT); Mauro Ajani, Milan (IT): Lorenzo Fossati, Milan (IT), CONTROLLED RELEASE AND TASTE MASKING ORAL PHARMACEUTICAL COMPOSITION., 2018, Cosmo Technologies Limited, Wicklow (IE). US patent 10660858B2,United States.
Schreiber S, Kamm MA, Lichtenstein GR. Mesalamine with MMX™ technology for the treatment of ulcerative colitis. Expert Rev Gastroenterol Hepatol. 2008;2(3):299–314.
Lichtenstein GR, et al. Effect of once-or twice-daily MMX mesalamine (SPD476) for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2007;5(1):95–102.
Hardy J, et al. Gastrointestinal transit of an enteric-coated delayed-release 5-aminosalicylic acid tablet. Aliment Pharmacol Ther. 1987;1(3):209–16.
Hardy J, Healey J, Reynolds J. Evaluation of an enteric-coated delayed-release 5-aminosalicylic acid tablet in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 1987;1(4):273–80.
Hardy J, et al. Localization of drug release sites from an oral sustained-release formulation of 5-ASA (Pentasa®) in the gastrointestinal tract using gamma scintigraphy. J Clin Pharmacol. 1993;33(8):712–8.
Healey J. Gastrointestinal transit and release of mesalazine tablets in patients with inflammatory bowel disease. Scand J Gastroenterol. 1990;25(sup172):47–51.
De Vos M, et al. Concentrations of 5-ASA and Ac-5-ASA in human ileocolonic biopsy homogenates after oral 5-ASA preparations. Gut. 1992;33(10):1338–42.
Naganuma M, et al. Measurement of colonic mucosal concentrations of 5-aminosalicylic acid is useful for estimating its therapeutic efficacy in distal ulcerative colitis: comparison of orally administered mesalamine and sulfasalazine. Inflamm Bowel Dis. 2001;7(3):221–5.
Ahmed I. Effect of simulated gastrointestinal conditions on drug release from pectin/ethylcellulose as film coating for drug delivery to the colon. Drug Dev Ind Pharm. 2005;31(4–5):465–70.
Layer PH, et al. Delivery and fate of oral mesalamine microgranules within the human small intestine. Gastroenterology. 1995;108(5):1427–33.
Hua Z, et al. Technology to obtain sustained release characteristics of drugs after delivered to the colon. J Drug Target. 1999;6(6):439–48.
Stolk L, et al. Dissolution profiles of mesalazine formulations in vitro. Pharm Weekbl. 1990;12(5):200–4.
Arkbage K, et al. Bioaccessibility of folic acid and (6 S)-5-methyltetrahydrofolate decreases after the addition of folate-binding protein to yogurt as studied in a dynamic in vitro gastrointestinal model. J Nutr. 2003;133(11):3678–83.
Tenjarla S, et al. Release of 5-aminosalicylate from an MMX mesalamine tablet during transit through a simulated gastrointestinal tract system. Adv Ther. 2007;24(4):826–40.
Schellekens R, et al. A novel dissolution method relevant to intestinal release behaviour and its application in the evaluation of modified release mesalazine products. Eur J Pharm Sci. 2007;30(1):15–20.
D’haens, G, . Systematic review: second-generation vs. conventional corticosteroids for induction of remission in ulcerative colitis. Aliment Pharmacol Therap. 2016;44(10):1018–29.
UCERIS®/ CORTIMENT®, https://www.cosmopharma.com/products/uceris-Cortiment. [cited 2021 Feb 20].
Roberto Villa , L.I.M.P., Gignese ( IT ) ; Mauro Ajani , Milan ( IT ) ; Lorenzo Fossati , Milan ( IT ) CONTROLLED RELEASE AND TASTE MASKING ORAL PHARMACEUTICAL COMPOSITION 2017, COSMO TECHNOLOGIES LIMITED , Dublin ( IE ).
De Vos M. Clinical pharmacokinetics of slow release mesalazine. Clin Pharmacokinet. 2000;39(2):85–97.
Gionchetti P, et al. Bioavailability of single and multiple doses of a new oral formulation of 5-ASA in patients with inflammatory bowel disease and healthy volunteers. Aliment Pharmacol Ther. 1994;8(5):535–40.
Brunner M, et al. Gastrointestinal transit, release and plasma pharmacokinetics of a new oral budesonide formulation. Br J Clin Pharmacol. 2006;61(1):31–8.
Malayandi R, et al. Biopharmaceutical considerations and characterizations in development of colon targeted dosage forms for inflammatory bowel disease. Drug Deliv Transl Res. 2014;4(2):187–202.
Farkas K, Molnár T. Novel extended release budesonide formulation for treatment of ulcerative colitis. Expert Opin Pharmacother. 2014;15(1):131–7.
COSMO AND SANTARUS FILE LAWSUIT AGAINST PAR FOR PATENT INFRINGEMENT OF UCERIS PATENTS. 2015 [cited 2015 February 3]; Available from: https://www.cosmopharma.com/news-and-media/press-releases-and-company-news/2015/03-02-2015.
Celasco G, et al. Efficacy of intracolonic administration of low-molecular-weight heparin CB-01-05, compared to other low-molecular-weight heparins and unfractionated heparin, in experimentally induced colitis in rat. Dig Dis Sci. 2008;53(12):3170–5.
Shen J, et al. Meta-analysis: the utility and safety of heparin in the treatment of active ulcerative colitis. Aliment Pharmacol Ther. 2007;26(5):653–63.
Celasco G, et al. Clinical trial: oral colon-release parnaparin sodium tablets (CB-01-05 MMX®) for active left-sided ulcerative colitis. Aliment Pharmacol Ther. 2010;31(3):375–86.
Pastorelli L, et al. Oral, colonic-release low-molecular-weight heparin: an initial open study of Parnaparin-MMX for the treatment of mild-to-moderate left-sided ulcerative colitis. Aliment Pharmacol Ther. 2008;28(5):581–8.
Chande N, et al. Unfractionated or low-molecular weight heparin for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews. 2015;10(8):6774.
Mauro Ajani, M.I.R.B., Milan (IT): Giuseppe Celasco, Genoa (IT); Roberto Villa, Lecco (IT), , ORAL ANTIMICROBIAL PHARMACEUTICAL COMPOSITIONS. 2013, US 8,486,446 B2, Cosmo Technologies Ltd., Dublin (IE).
Riddle MS, Connor BA, Tribble DR. Targeted Therapy in Travelers’ Diarrhea: What Is the Role for the Non-Absorbable? Oxford: Oxford University Press; 2014.
Steffen R, et al. Rifamycin SV-MMX® for treatment of travellers’ diarrhea: equally effective as ciprofloxacin and not associated with the acquisition of multi-drug resistant bacteria. Oxford: Oxford University Press; 2018.
Di Stefano A, et al. Systemic absorption of rifamycin SV MMX administered as modified-release tablets in healthy volunteers. Antimicrob Agents Chemother. 2011;55(5):2122–8.
Connor BA. A Closer Look at AEMCOLO With MMX Technology for the Treatment of Travelers’ Diarrhea. Gastroenterol Hepatol. 2019;15(2):1.
Shayto RH, Abou Mrad R, Sharara AI. Use of rifaximin in gastrointestinal and liver diseases. World J Gastroenterol. 2016;22(29):6638.
DuPont HL, et al. Targeting of rifamycin SV to the colon for treatment of travelers’ diarrhea: a randomized, double-blind, placebo-controlled phase 3 study. J Travel Med. 2014;21(6):369–76.
Duncan MB, et al. Use of methylene blue for detection of specialized intestinal metaplasia in GERD patients presenting for screening upper endoscopy. Dig Dis Sci. 2005;50(2):389–93.
Di Stefano A, et al. Methylene blue MMX® tablets for chromoendoscopy. Bioavailability, colon staining and safety in healthy volunteers undergoing a full colonoscopy. Contemp Clin Trials. 2018;71:96–102.
Repici A, et al. Methylene blue MMX® tablets for chromoendoscopy. Safety tolerability and bioavailability in healthy volunteers. Contemporary clinical trials. 2012;33(2):260–7.
Vermeire S, Rutgeerts P. Current status of genetics research in inflammatory bowel disease. Genes Immun. 2005;6(8):637–45.
Camerini R. Can we treat ulcerative colitis with nutritional supplements? author’s reply. Aliment Pharmacol Ther. 2012;35(4):486–7.
Wagner CC, et al. Plasma pharmacokinetics and gastrointestinal transit of a new Propionyl-l-Carnitine controlled release formulation. Xenobiotica. 2011;41(11):988–95.
Ghate VM, Chaudhari P, Lewis SA. Physiologically based pharmacokinetic (PBPK) modelling for in vitro-in vivo extrapolation: emphasis on the use of dissolution data. Dissolut Technol. 2019;26(03):18–27.
Mishra V, et al. Quality by design (QbD) approaches in current pharmaceutical set-up. Expert Opin Drug Deliv. 2018;15(8):737–58.
Grangeia HB, et al. Quality by design in pharmaceutical manufacturing: A systematic review of current status, challenges and future perspectives. Eur J Pharm Biopharm. 2020;147:19–37.
Venkateshwaran HG. Successful Process Analytical Technology (PAT) implementation in pharmaceutical manufacturing. European Pharmaceutical Review, 2008;(5). https://www.europeanpharmaceuticalreview.com/article/1485/successful-pat-implementation-pharmaceutical-manufacturing/
Shah RB, Tawakkul MA, Khan MA. Process analytical technology: chemometric analysis of Raman and near infra-red spectroscopic data for predicting physical properties of extended release matrix tablets. J Pharm Sci. 2007;96(5):1356–65.
Lukacova V, Woltosz WS, Bolger MB. Prediction of modified release pharmacokinetics and pharmacodynamics from in vitro, immediate release, and intravenous data. AAPS J. 2009;11(2):323–34.
Kesisoglou F, Balakrishnan A, Manser K. Utility of PBPK absorption modeling to guide modified release formulation development of gaboxadol, a highly soluble compound with region-dependent absorption. J Pharm Sci. 2016;105(2):722–8.
Shadle, C., et al. Assessment of dose proportionality, absolute bioavailability, and tolerability of gaboxadol in healthy young adults. in Sleep. 2006. Amer Academy Sleep Medicine One Westbrook Corporate Center Ste 920 ….
Crison JR. Physiologically Based Pharmacokinetic Modeling in the Development and Evaluation of Hydrophilic Matrix Tablets. In: Hydrophilic Matrix Tablets for Oral Controlled Release. Springer; 2014. p. 191–203.
Basu S, et al. Physiologically based pharmacokinetic modeling to evaluate formulation factors influencing bioequivalence of metoprolol extended-release products. J Clin Pharmacol. 2019;59(9):1252–63.
Abdul S, Chandewar AV, Jaiswal SB. A flexible technology for modified-release drugs: multiple-unit pellet system (MUPS). J Control Release. 2010;147(1):2–16.
Wagner KG, et al. Development of disintegrating multiple-unit tablets on a high-speed rotary tablet press. Eur J Pharm Biopharm. 2000;50(2):285–92.
Vo Anh Q, et al. "Application of FT-NIR analysis for in-line and real-time monitoring of pharmaceutical hot melt extrusion: a technical note." Aaps Pharmscitech 2018;19(8):3425–3429.
Mundada PK, Sawant KK, Mundada VP. Formulation and optimization of controlled release powder for reconstitution for metoprolol succinate multi unit particulate formulation using risk based QbD approach. Journal of Drug Delivery Science and Technology. 2017;41:462–74.
Sharma, Kapil Dev, and Shobhit Srivastava. "Failure mode and effect analysis (FMEA) implementation: a literature review." J Adv Res Aeronaut Space Sci 2018;5:1–17.
Charoo NA, et al. Quality by design approach for formulation development: a case study of dispersible tablets. Int J Pharm. 2012;423(2):167–78.
Fahmy R, Danielson D, Martinez MN. Quality by design and the development of solid oral dosage forms. In: Long Acting Animal Health Drug Products. Springer; 2013. p. 107–29.
Singh G, Pai RS, Devi VK. Optimization of pellets containing solid dispersion prepared by extrusion/spheronization using central composite design and desirability function. J Young Pharm. 2012;4(3):146–56.
Pabari RM, Ramtoola Z. Application of face centred central composite design to optimise compression force and tablet diameter for the formulation of mechanically strong and fast disintegrating orodispersible tablets. Int J Pharm. 2012;430(1–2):18–25.
Desai D, et al. Formulation design, challenges, and development considerations for fixed dose combination (FDC) of oral solid dosage forms. Pharm Dev Technol. 2013;18(6):1265–76.
Lee B-J, Ryu S-G, Cui J-H. Controlled release of dual drug-loaded hydroxypropyl methylcellulose matrix tablet using drug-containing polymeric coatings. Int J Pharm. 1999;188(1):71–80.
Robinson JR, Lee VH. Controlled drug delivery: fundamentals and applications/edited by Joseph R Robinson, Vincent HL Lee. New York: Dekker; 1987.
Rathore AS, et al. An overview: Matrix tablet as controlled drug delivery system. Int J Res Dev Pharm Life Sci. 2013;2(4):482–92.
Nellore RV, et al. Development of metoprolol tartrate extended-release matrix tablet formulations for regulatory policy consideration. J Control Release. 1998;50(1–3):247–56.
Skelly J, et al. Scale-up of oral extended-release dosage forms. Pharm Technol. 1995;19(5):46–54.
FDA. SUPAC-MR: Modified Release Solid Oral Dosage Forms Scale-Up and Postapproval Changes: Chemistry, Manufacturing, and Controls; In Vitro Dissolution Testing and In Vivo Bioequivalence Documentation. 2020 [cited 2020 Nov 2]; Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/supac-mr-modified-release-solid-oral-dosage-forms-scale-and-postapproval-changes-chemistry.
FDA. Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations. 2020 [cited 2020 Nov 2]; Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/extended-release-oral-dosage-forms-development-evaluation-and-application-vitroin-vivo-correlations.
Pouillon L, et al. Head-to-head trials in inflammatory bowel disease: Past, present and future. Nat Rev Gastroenterol Hepatol. 2020;17(6):365–76.
McConnell EL, Murdan S, Basit AW. An investigation into the digestion of chitosan (noncrosslinked and crosslinked) by human colonic bacteria. J Pharm Sci. 2008;97(9):3820–9.
Abd Elbary A, Aboelwafa AA, Al Sharabi IM. Once daily, high-dose mesalazine controlled-release tablet for colonic delivery: optimization of formulation variables using Box-Behnken design. Aaps Pharmscitech. 2011;12(4):1454–64.
Guideline IHT. Stability testing of new drug substances and products. Q1A (R2) Curr Step. 2003;4:1–24.
Simoni SE, et al. Multi-Matrix 5-Aminosalicylic Acid Efficacy in Induction of Remission in Mild-to-Moderate Ulcerative Colitis: A Systematic Review. Int J Health Sci. 2019;7(3):42–51.
Atsuo Kitano S. Japan, 6,025,393, Method For Treatment Of Inflammatory Intestinal Diseases. Osaka: Santen Pharmaceutical Co. Ltd; 2000.
Acknowledgements
This is NIPER Hyderabad manuscript No.: NIPER-H/2020/M071.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sriram, A., Tangirala, S., Atmakuri, S. et al. Budding Multi-matrix Technology—a Retrospective Approach, Deep Insights, and Future Perspectives. AAPS PharmSciTech 22, 264 (2021). https://doi.org/10.1208/s12249-021-02133-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02133-4