Abstract
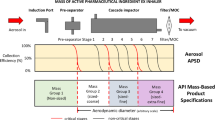
This study of aerodynamic mass-weighted particle size distribution (APSD) data from orally inhaled products (OIPs) investigated whether a set of simpler (than currently used) metrics may be adequate to detect changes in APSD for quality control (QC) purposes. A range of OIPs was examined, and correlations between mass median aerodynamic diameter and the ratio of large particle mass (LPM) to small particle mass (SPM) were calculated. For an Andersen cascade impactor, the LPM combines the mass associated with particle sizes from impactor stage 1 to a product-specific boundary size; SPM combines the mass of particles from that boundary through to terminal filter. The LPM–SPM boundary should be chosen during development based on the full-resolution impactor results so as to maximize the sensitivity of the LPM/SPM ratio to meaningful changes in quality. The LPM/SPM ratio along with the impactor-sized mass (ISM) are by themselves sufficient to detect changes in central tendency and area under the APSD curve, which are key in vitro quality attributes for OIPs. Compared to stage groupings, this two-metric approach provides better intrinsic precision, in part due to having adequate mass and consequently better ability to detect changes in APSD and ISM, suggesting that this approach should be a preferred QC tool. Another advantage is the possibility to obtain these metrics from the abbreviated impactor measurements (AIM) rather than from full-resolution multistage impactors. Although the boundary is product specific, the testing could be accomplished with a basic AIM system which can meet the needs of most or all OIPs.








Similar content being viewed by others
Abbreviations
- ACI:
-
Andersen cascade impactor
- AIM:
-
abbreviated impactor measurement
- API:
-
active pharmaceutical ingredient
- APSD:
-
aerodynamic particle size distributions
- AUCAPSD :
-
area under the APSD curve
- CI:
-
cascade impaction
- CQA:
-
critical quality attribute
- DPI:
-
dry powder inhaler
- GSD:
-
geometric standard deviation
- HRT:
-
human respiratory tract
- IPAC-RS:
-
International Pharmaceutical Aerosol Consortium on Regulation and Science
- ISM:
-
impactor-sized mass (defined as mass on all impactor components with a defined upper cutoff, i.e., from stage 1 to filter)
- LPM:
-
large particle mass (defined uniquely for each product, from stage 1 to some defined boundary within the impactor)
- MDI:
-
metered dose inhaler
- MMAD:
-
mass median aerodynamic diameter
- MMF:
-
Morgan–Mercer–Flodin
- NGI:
-
next generation pharmaceutical impactor
- OIP:
-
orally inhaled product
- QC:
-
quality control
- RMSE:
-
root mean square error
- SPM:
-
small particle mass (defined uniquely for each product, from some defined boundary within the impactor to the filter)
References
FDA. Guidance for industry PAT—a framework for innovative pharmaceutical development, manufacturing, and quality assurance. 2004. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070305.pdf. Accessed 4 Aug 2009.
FDA CDER. Draft guidance for industry metered dose inhaler (MDI) and dry powder inhaler (DPI) drug products chemistry, manufacturing, and controls documentation. 1998. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070573.pdf. Accessed 4 Aug 2009.
EP 2.9.18. Preparations for inhalations: aerodynamic assessment of fine particles. Strasbourg: Council of Europe; 2008.
USP 31. Chapter <601> aerosols, nasal sprays, metered-dose inhalers, and dry powder inhalers. Rockville: USP; 2008.
Health Canada. Pharmaceutical quality of inhalation and nasal products. 2006. http://www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/chem/inhalationnas_e.html (Accessed 4 Aug 2009) and EMEA. 2006 EMEA/CHMP/QWP/49313/2005 Corr. http://www.emea.europa.eu/pdfs/human/qwp/4931305en.pdf (Accessed 4 Aug 2009). Also adopted by Australia: http://www.tga.gov.au/docs/pdf/euguide/qwp/4931305en.pdf. Accessed 4 May 2009.
Adams WP, Christopher D, Lee DS, Morgan B, Pan Z, Singh GJP, Tsong Y, Lyapustina S. Product Quality Research Institute evaluation of cascade impactor profiles of pharmaceutical aerosols, part 1: background for a statistical method. AAPS PharmSciTech. 2007;8(1):Article 4. doi:10.1208/pt0801004. http://www.aapspharmscitech.org/default/issueView.asp?vol=08&issue=01 or http://www.aapspharmscitech.org/view.asp?art=pt0801004. Accessed 4 Aug 2009.
Mitchell JP, Dunbar C. Analysis of cascade impactor mass distributions. J Aerosol Med. 2005;18(4):439–51.
Tougas T. Capabilities of aerodynamic particle size distribution (APSD) measurements based on analysis of a blinded database. RDD. 2008;1:109–23.
Christopher D, Curry P, Doub W, Furnkranz K, Lavery M, Lin K, et al. Considerations for the development and practice of cascade impaction testing including a mass balance failure investigation tree. J Aerosol Med. 2003;16(3):235–47.
Van Oort M, Roberts W. Variable flow–variable stage–variable volume strategy for cascade impaction testing of inhalation aerosols. In: Dalby RN, Byron PR, Farr SJ, editors. Respiratory Drug Delivery V. Buffalo Grove: Interpharm; 1996. p. 418–20.
Lundbäck H, Wiktorsson B. High throughput inhaler testing I: fine particle dose. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, editors. Respiratory Drug Delivery 2006. River Grove: Davis Healthcare International; 2006. p. 467–9.
Initial assessment of the ITFG/IPAC aerodynamic particle size distribution database by the CMC specifications technical team of the ITFG/IPAC collaboration. 2000. http://ipacrs.com/PDFs/Initial_Assess_of_Particle.PDF. Accessed 4 Aug 2009.
Morgan PH, Mercer LP, Flodin NW. General model for nutritional responses of higher organisms. Proc Natl Acad Sci USA. 1975;72:4327–31.
Christopher D, Dey M, Lyapustina S, Mitchell JP, Stein S, Tougas TP, Van Oort M, Strickland H, Wyka B. Generalized simplified approaches for MMAD determination. Pharmacop Forum. 2009; in press.
Christopher D, Dey M, Lyapustina S, Mitchell J, Stein S, Tougas T, Van Oort M. Alternative approaches for MMAD determination. Poster presented at the IPAC-RS Conference 2008. http://ipacrs.com/PDFs/Posters/Alternative%20MMAD.pdf. Accessed 4 Aug 2009.
Marple VA, Willeke K. Inertial impactors: theory, design and use. In: Liu BYH, editor. Fine Particles. New York: Academic; 1976. p. 411–66.
Kamiya A, Sakagami M, Hindle M, Byron PR. Aerodynamic sizing of metered dose inhalers: an evaluation of the Andersen and next generation pharmaceutical impactors and their USP methods. J Pharm Sci. 2004;93(7):1828–37.
Mitchell JP, Nagel MW, Wiersema KJ, Doyle CC. Aerodynamic particle size analysis of aerosols from pressurized metered dose inhalers: comparison of Andersen 8-stage cascade impactor, next generation pharmaceutical impactor, and model 3321 aerodynamic particle sizer aerosol spectrometer. AAPS PharmSciTech. 2003;4(4):article 54.
Mitchell JP, Nagel MW, Avvakoumova V, MacKay H, Ali R. The abbreviated impactor measurement (AIM) concept: part I—influence of particle bounce and re-entrainment—evaluation with a “dry” pressurized metered dose inhaler (pMDI)-based formulation. AAPS PharmSciTech. 2009;10(1):243–51.
Mitchell JP, Nagel MW, Avvakoumova V, MacKay H, Ali R. The abbreviated impactor measurement (AIM) concept: part II—influence of evaporation of a volatile component—evaluation with a “droplet-producing” pressurized metered dose inhaler (pMDI)-based formulation containing ethanol as cosolvent. AAPS PharmSciTech. 2009;10(1):252–7.
Acknowledgments
The authors appreciate the ongoing support of the IPAC-RS Board of Directors for this project, comments from the IPAC-RS Cascade Impaction Working Group, and technical advice from the European Pharmaceutical Aerosol Group. Our appreciation for help in preparing this manuscript is also extended to Samantha Dickinson. Finally, we also wish to acknowledge the technical advice from Mårten Svensson of AstraZeneca, Lund, Sweden, regarding AIM options.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tougas, T.P., Christopher, D., Mitchell, J.P. et al. Improved Quality Control Metrics for Cascade Impaction Measurements of Orally Inhaled Drug Products (OIPs). AAPS PharmSciTech 10, 1276–1285 (2009). https://doi.org/10.1208/s12249-009-9312-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-009-9312-4