Abstract
Controversy exists as to whether the parenterally (PN) fed human neonate is capable of synthesizing adequate cysteine from methionine if the total dietary requirement for sulfur amino acid (SAA) is provided as methionine only. The goal of this study was to gather data on whether glutathione (GSH) synthesis is maximized at a methionine intake previously shown to be adequate for protein synthesis in the PN-fed human neonate. We measured GSH concentration, fractional, and absolute synthesis rate in five PN-fed human neonates. Each neonate underwent two isotope infusion studies of 7 h duration after a 2-d adaptation to the total SAA requirement (methionine only) and again after a further 2-d adaptation to the same methionine intake supplemented with cysteine at 10 mg · kg−1 · d−1. Cysteine supplementation did not significantly affect GSH synthesis. These data suggest that term infants are capable of synthesizing cysteine from methionine, not only for protein but also for GSH synthesis.
Similar content being viewed by others
Main
The suggestion that cysteine might be an indispensable amino acid (AA) in preterm and term neonates was first introduced by Gaull and coworkers (1), who showed absence of cystathionase activity in the liver of premature fetuses and newborns. This report was later confirmed by the same investigators in subsequent experiments (2,3). Other investigators using differing endpoints have come to similar conclusions. Vina et al. (4), using plasma cysteine concentrations and in vitro erythrocyte [glutathione (GSH)] synthesis, concluded that neonates ≤32-wk gestation had lower plasma cysteine and slower GSH synthesis than neonates >33-wk gestation. In addition, others have reported low plasma cysteine and high plasma methionine in neonates fed low to cysteine-free parenteral nutrition (PN) (5–7) compared with breast fed neonates. These observations have been used as evidence that cysteine is an indispensable AA for the human neonate.
Conversely, later studies evaluating cystathionase activity, in liver, kidney, adrenals, and pancreas of infants who died before 1 y of age concluded that cystathionase activity in the term and premature infant was considerably greater than previously appreciated; and that if the total sulfur AA (SAA) requirement was adequate, even provided as methionine only, cysteine may not be a concern (8). The same group (9) and others (10) found that when cysteine was supplemented to cysteine-free PN, there was no improvement in growth and nitrogen balance in the supplemented group with both groups achieving similar to in utero nitrogen retention. However, other reports have concluded that although growth was not affected, nitrogen balance was increased with cysteine supplementation (11). More recent stable isotope data showed significant transfer of label from D-[U-13C]glucose into cysteine derived from (apo) B-100 in preterm infants fed cysteine-free PN (12). This showed that preterm infants had the ability to synthesis cysteine de novo. More recently, data in enterally fed neonates have provided additional convincing evidence that cysteine is not indispensable even in the very low birth weight, preterm infant (13,14). All of these data however, relate to the neonate's ability to synthesize cysteine for protein synthesis and may not extend to cysteine synthesis in amounts adequate for GSH synthesis.
Vina et al. (4) showed that GSH synthesis is slow in the preterm neonate. Zlotkin and Anderson (15) were unable to account for all of the cysteine supplemented to neonates receiving cysteine-free PN and Shew et al. (12), although able to show cysteine synthesis in preterm neonates, also showed that there was a significant positive relationship between synthesis and neonatal age. These data provide evidence that the pathway may be underdeveloped in the neonate, especially those who are premature. The unanswered question therefore, is whether the minimum synthetic capacity for cysteine observed in the neonate is sufficient to maintain GSH status if SAA is provided as methionine only. This question was partly answered in a recent study showing no difference in GSH synthesis between neonates receiving i.v. glucose or those receiving glucose plus AAs (16).
The goal of this study, therefore, was to determine whether supplemental cysteine would result in a GSH synthesis rate higher than that observed when the total SAA was provided at requirement (17) but as methionine only in the PN-fed human neonate.
METHODS
Subjects.
Five neonates were enrolled in this study. The following inclusion criteria were used to determine eligibility: lack of chromosomal anomalies, born at ≥34-wk gestation and ≤28-d chronological age at the time of the study, birth weight and length appropriate for gestational age, medically stable as determined by normal blood results and lack of a fever or infection, at least 3 d postoperatively, and on PN providing adequate protein and calories. Exclusion criteria included small for gestational age, presence of disease or on medications known to affect protein and AA metabolism, documented infection, fever, unstable medical condition, and receiving enteral feeding providing >10% of protein intake.
Ethical approval for the study was obtained from The Research Ethics Board at The Hospital for Sick Children. Permission was obtained from the attending physician before approaching parents, and written informed consent was obtained from at least one parent before enrolling subjects into the study. Study characteristics of the neonates included in the study is presented in Table 1.
Experimental design.
The precursor product model developed by Jahoor et al. (18) was used to determine GSH kinetics in this study. Each neonate was studied over 6 d. The study was divided in two parts. In part 1, subjects received a parenteral AA solution devoid of cysteine, with the total SAA requirements provided as methionine only. In part 2, the solution was similar to that used in part 1 but contained a supplemental cysteine at 10 mg/kg. We did not randomize the subjects to the order of cysteine intake because the length of the study was very short; differences in maturity would be too small to measure over a 6-d period. This protocol also made it easier to ensure that the babies did not receive enteral feeds during the cysteine-free part on the study.
During the first 3 d, neonates received an AA solution (solution 1) patterned after a commercial AA base solution (Primene, Baxter Laboratories, Mississauga, Ontario) (Table 2), plus dextrose, and a 20% lipid solution (Intralipid; Fresenius Kabi, Uppsala, Sweden) for provision of adequate protein, and nonprotein energy. Standard amounts of vitamin and minerals were provided in the form of a liquid supplement (Multi-12/K1, providing a mixture of fat and water-soluble vitamins) formulated for use in i.v. feeding. All vitamins and minerals met these dietary reference intakes (DRI) recommendations. The SAA in this solution was provided as methionine only at the recommended dietary allowance (RDA = 56 mg/kg) as previously determined in our laboratory (17). On the third day, a primed, continuous 7 h tracer infusion was carried out to measure GSH metabolism.
On d 4–6, neonates received a similar AA solution as that received on d 1–3 but with supplemental cysteine as cysteine.HCl, providing cysteine at an intake of 10 mg · kg−1 · d−1 (solution 2) (Table 2). Intakes of dextrose, lipid, vitamins, minerals, total calories, and protein were kept the same as on the previous 3 d. On the sixth d, a second primed, continuous 7 h tracer infusion study was conducted to measure GSH kinetics with additional cysteine.
Given the complexity and length of this study, it was very difficult to recruit babies. The chief problem was that of enrolling babies that would be stable postoperatively for 6 d on PN and not advance to significant amounts of enteral feeds (>10% of protein enterally). Two additional babies that began the study had to be withdrawn because of advancement to large volumes of enteral feeds.
Study diets.
The amino profile of the AA solution used in the study (solutions 1 and 2) is presented in Table 2. The composition was patterned after a commercial AA solution with an AA composition of umbilical cord blood (base AA solution). The AA solutions used were prepared using human pharmacy grade AAs (Ajinomoto Company Inc., Japan via L.V Lomas, Brampton, Ontario) in our research laboratory, under sterile conditions. The profile of the AA base was followed with some modifications; methionine was varied to provide the requirement estimate derived previously by our laboratory (17) (56 mg · kg−1 · d−1), cysteine was removed from solution 1 but added to solution 2 at 10 mg · kg−1, tyrosine was provided as the dipeptide glycyl- tyrosine at a level of 4 g/100 g (19). Arginine was increased from 8.4 to 9.66 g/100 g based on our piglet studies (20,21), and aspartate was decreased from 6.0 to 5.0 g/100 g to accommodate for the increased amount of nitrogen provided by arginine. Alanine was used to balance the nitrogen and make the solutions isonitrogenous. Alanine was prepared as a separate test solution at a concentration of 50 mg · mL−1. All prepared solutions were filter sterilized in the Research Pharmacy at the Hospital for Sick Children by being passed though a 0.22-μm filter. Solutions were subsequently demonstrated to be sterile and free of bacterial growth over 7 d in culture and proven to be pyrogen free by the Limulus amebocyte lysate test (22). The chemical composition of the solutions was verified by AA analysis using HPLC and analysis of total nitrogen.
All subjects were receiving adequate protein and energy (23,24). Nonprotein energy was provided as dextrose and a 20% lipid solution. All calculations of nutrient intake for phase 1 of the study (d 1–3) were done using body weight on d 1 of the study, whereas calculation of nutrient intake for phase 2 (d 4–6) were done using weight on d 4 of the study. Mean body weight of the subjects was not different on d 1 versus d 4.
The actual intake of the subjects differed slightly from the prescription because the volume of solution prescribed is not always that which is delivered in a clinical environment.
Tracer protocol.
[U-13C2-15N]glycine (98% 13C2, 98% 15N) (Cambridge Isotope Laboratories, Andover, MA) was used for the measurement of erythrocyte GSH synthesis. Stock solutions were prepared in 0.9% sodium chloride (10 mg/mL) by the Research Pharmacy at the Hospital for Sick Children, Toronto, Canada and were confirmed to be sterile and pyrogen free. Infusion solutions were aliquoted into sterile bottles and kept in the Research Pharmacy at the Hospital for Sick Children, Toronto, Canada at 4°C until use.
An i.v. priming dose of [U-13C2 –15N] glycine was given at 50 μmol · kg−1 over 15 min followed by a continuous i.v. infusion of 40 μmol · kg−1 · hr−1 for a total of 7-h isotope infusion time.
We chose to use an M + 3 glycine tracer as the GSH precursor and determined its incorporation into the GSH molecular ion by LCMS/MS. The reason for our choice has been previously described (25).
Sample collection.
Blood samples were collected on d 3 and 6 at baseline, before the start of the isotope infusion, for analysis of background enrichment. A total of 0.7 mL of blood was collected at each time point; 0.5 mL for measurement of erythrocyte GSH concentration and enrichment and plasma AA concentration, and 0.2 mL for the measurement of erythrocyte intracellular glycine enrichment; which is the precursor pool from which glycine is drawn for GSH synthesis. Having previously established that isotopic steady state was reached in the precursor pool at 5 h, two subsequent blood samples were taken at ∼5 ½ and 6 ½ h. Hematocrit was obtained from blood samples collected for clinical monitoring on the same day of the isotope study. Baseline blood work, performed for clinical monitoring, was reviewed on each subject before the start of the study. All subjects had normal sodium, potassium, calcium, phosphorous, and pH.
Erythrocyte GSH concentration and enrichment.
All chemicals were purchased from Sigma Chemical Co.-Aldrich Canada Ltd., Oakville, Ontario, Canada. A 0.5-mL aliquot of each blood sample collected in Na2EDTA was centrifuged for 2 min within 5 min of collection. After centrifugation, the plasma was immediately removed. Two hundred micro liters of 100 mM N-ethylmaleimide, and 20 μL of 5 mM γ-glutamyl-leucine (internal standard) were added to the separated red blood cells. The sample was then caped, vortex for 2 min, and left for 10 min at room temperature. Cells were then lysed with 50 μL 0.4 M ZnSO4, and the protein was precipitated with 1 mL ice cold methanol. The sample was then vortexed, centrifuged for 2 min, the supernatant was removed, and stored at −80°C until analyzed.
GSH concentration and enrichment were analyzed as previously described (25). Briefly, GSH concentration was measured using an external standard curve and the ratio of the analyte (GSH) to the internal standard (γ-glutamyl-leucine). GSH enrichment was calculated as a ratio of the (enriched) M + 3 to (unenriched) M peaks of the tripeptide molecule of GSH and was expressed as mole percent excess and was calculated from peak area ratios at isotopic steady state of glycine in the last 2 h of isotope infusion.
Erythrocyte free glycine enrichment.
Each sample was collected and centrifuged as earlier. Plasma was quickly removed, and the cells were washed twice with iced cold saline. Samples were vortexed between each wash. Cells were then lysed and deproteinated as earlier, vortexed, centrifuged for 2 min, and the supernatant was stored at −80°C until analysis.
Fifty microliters of each sample was then dried under nitrogen at 35°C. One hundred microliters of butanol HCl (Sigma Chemical Co.-Aldrich Canada Ltd.) was then added and the sample was vortexed topped with nitrogen and heated for 20 min at 55°C. The sample was again dried under nitrogen and reconstituted in 0.1% formic acid (Sigma Chemical Co.-Aldrich Canada Ltd.). Samples were analyzed using a triple quadruple mass analyzer as previously described (25). Glycine enrichment was calculated as a ratio of the (enriched) M + 3 to (unenriched) M peaks using peak area ratios at isotopic steady state and expressed as mole percent excess above baseline.
Plasma AA concentration.
Plasma total cysteine concentration was determined by LCMS/MS, using a bench top triple quadruple mass spectrometer API 4000 (Applied Biosystems/MDS SCIEX) operated in positive ionization mode with the Turbo Ion Spray ionization probe source (operated at 5.8 KV). This was coupled to an Agilent 1100 HPLC system. Measurements were as previously described (26).
Fractional and absolute synthesis rate of erythrocyte GSH.
The fractional synthesis rate (FSR) of erythrocyte GSH (FSRGSH) was calculated using the precursor-product method of Jahoor et al. (18).
Equation

where (Et6.5 − Et5.5) was the increase in the isotopic enrichment of erythrocyte GSH between 5 ½ and 6 ½ h of infusion as a result of the incorporation of the labeled glycine, ERBC was the intracellular glycine enrichment at isotopic steady state, and (t 6.5 − t 5.5) was the time interval between the 5 ½ and 6 ½ h when the incorporation of glycine into GSH was measured.
Equation

where GSHmass = the product of the cell volume (or cell number or cell protein) and the concentration of GSH in the cell. Hematocrit was calculated using the formula; HCT (L/L) = (RBC × MCV)/1000.
Statistical analysis.
The data were analyzed by repeated measures ANOVA with the PROC GLM procedure to assess the effect of cysteine intake on GSH concentration, FSR, ASR, and cysteine concentration. Independent variables tested were cysteine intake and subject. Because order of cysteine intake was not randomly assigned, order was not included in the model. Statistical significance was established at p ≤ 0.05. Data were analyzed by SAS version 9.1 for Windows (SAS Institute Inc., Cary, NC).
RESULTS
Clinical characteristics and nutrient intake.
The clinical characteristics and diagnosis of the five subjects studied are presented in Table 1. The total nutrient intake was dependent on the total volume of PN infused. The average methionine intake for phases 1 and 2 was 60.3 ± 3.7 and 60.5 ± 2.0 mg · kg−1 · d−1, respectively, whereas the cysteine intake in phase 1 was 0 mg · kg−1 · d−1 by design and 10.3 mg · kg−1 · d−1 in phase 2. The average energy and protein intake for phase 1 was 84 ± 4.0 kcals/kg and 3.1 ± 0.2 g · kg−1 · d−1, respectively, and was not different for phase 2, with energy intake being 86 ± 4.0 kcals · kg−1 · d−1 and protein being 3.1 ± 0.1 g · kg−1 · d−1.
AA concentration.
Cysteine concentration was numerically higher with cysteine supplementation, 152.5 ± 43.5 (mean ± SD) versus 173.7 ± 53.0 μmol/L−1, but the difference was not statistically significant.
Intracellular glycine enrichment.
Isotopic steady state (plateau) in the intracellular glycine pool was achieved for all neonates by 5 h after the start of the isotope infusion and was defined by the absence of a significant slope between the data points at plateau.
GSH kinetics.
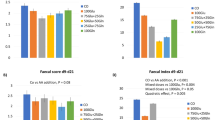
These are presented in Figure 1. The additional 10 mg · kg−1 · d−1 of cysteine had no effect on GSH concentration, fractional, or absolute synthesis rates (ASR). During the isotope infusion study on subject number 5, the baby was very fussy; therefore, during the blood collection at isotopic steady state, the father only permitted a blood sample to be taken at 5.5 h and not a second sample at 6.5 h. Hence the calculation of GSH FSR from the incorporation of tracer at the isotopic steady state of glycine could only be done for four of the five babies. The individual estimates of GSH FSR for those four babies are also shown in Figure 2. Rather than discard the data from subject 5, we also did a calculation of GSH FSR based on glycine tracer incorporation from baseline to 5.5 h (data not shown). For both estimates, there was not change in GSH FSR with the increase in cysteine intake of 10 mg · kg−1 · d−1.
Mean (± SD) effect of cysteine supplementation on (A) GSH concentration (n = 5), (B) fractional synthesis rate of GSH, and (C) the ASRs of GSH for four of five subjects who participated in the study, measured at isotopic steady state. Using repeated measures two-way ANOVA with PROC GLM procedure, cysteine supplementation had not effect on GSH concentration (0.19), FSRGSH (p = 0.18), or ASRGSH (p = 0.16).
DISCUSSION
This is the first study to our knowledge, conducted in the PN-fed human neonate to determine whether the provision of the total dietary SAA requirement (17) as methionine is also adequate for maintenance of GSH status. The main goal was to directly determine whether supplementation of cysteine to a PN solution providing the total SAA requirement as methionine only would stimulate an increase in GSH synthesis and concentration. Indirectly, the secondary goal was to assess whether the trans-sulfuration pathway was capable of synthesizing adequate cysteine from methionine, thereby providing further clarification as to whether cysteine is an indispensable AA in the PN-fed human neonate.
These results show that cysteine supplementation did not affect GSH concentration. Further, these concentrations are comparable with that obtained in neonates receiving PN (16) and that obtained from venous blood taken from the umbilical cord immediately after delivery in term babies (27) but was slightly lower than that observed in 20-mo-old enterally fed children treated for and recovered from malnutrition (28).
We chose a within subject design as the best approach to show the effect of cysteine supplementation should one occur. Because of the anticipated difficulty in the conduct of such a study, we chose to recruit five infants as a means of obtaining data for a future supplementation trial. Unfortunately, we were unable to measure GSH synthesis at steady state in one of the five subjects. As a result, we measured GSH synthesis in two different ways; in four of the five subjects at steady state and in all 5 subjects as incorporation over 5 ½ h. At steady state, the GSH FSR was higher than that measured over the course of the 5 ½ h; however, there was no effect of cysteine supplementation on GSH synthesis regardless of method used.
One of the important limitations of this study is the small sample size, thereby limiting the confidence required to draw definitive conclusions. Because of the difficulty inherent in this study design, namely the recruitment of babies for a 6-d study in which blood is drawn at least three times on 2 different days, a future design in which babies are randomized to cysteine supplementation or no supplementation in the presence of the current methionine intake should be more feasible. Nevertheless, a look at the individual data (Fig. 2) shows no sign of any trend toward an increase in GSH FSR with cysteine supplementation at 10 mg · kg−1 · d−1. In addition, a recently published report (16) suggested a sample size of ≥3 subjects to be adequate to show a difference in GSH synthesis rates. Therefore, the suggestion is that the four subjects used in this study represent an adequate sample size to show a change in GSH synthesis with cysteine supplementation if one occurred.
These data are supported by previous isotope data, which shows that preterm neonates are capable of cysteine synthesis, as evidenced by incorporation of 13C label from [13C6] glucose into apo B-100 cysteine (12) and more recent isotope date showing that there is significant trans-sulfuration of methionine in the neonate (29) whether fed enterally or parenterally. The results of this study indicate that not only are neonates capable of cysteine synthesis for adequate growth and nitrogen balance (9,10) but also cysteine synthesis from methionine is adequate for maintenance of GSH synthesis.
Another important consideration is whether at 10 mg · kg−1 · d−1 the level of cysteine supplementation was adequate to detect a significant change in GSH kinetics if one occurred. This level was chosen because of the concern for acid-base balance in the babies with the form of cysteine being cysteine.HCL. A previous cysteine supplementation study was able to show a statistically significant 25% increase in GSH FSR when cysteine was supplemented at 15 mg · kg−1 · d−1 in the presence of an adequate methionine intake of 28–33 mg · kg−1 · day−1 (30). In this study, with the total SAA requirement provided at the RDA as methionine (17), and knowledge that babies are able to synthesize adequate cysteine for proteins synthesis from methionine (9,31), and that cysteine is not an essential AA in the neonate (13,14), the addition of cysteine as a supplement, therefore, would be used for GSH synthesis if GSH synthesis was inadequate. Consequently, we believed that a supplemental intake of 10 mg · kg−1 · d−1 was adequate to detect a change in GSH FSR if one occurred.
An important finding in this study was the plasma cysteine concentrations without and with cysteine supplementation were 152.5 ± 43.7 and 173.7 ± 53.0 μmol/L, respectively; these were not statistically different. The plasma cysteine concentration, without supplemental cysteine, was similar to that observed in term breast fed babies (32), which suggests that when the SAA are provided at an adequate intake and as methionine only, PN-fed human neonates are able to synthesize adequate cysteine from methionine for cysteine homeostasis.
This is the first study, to our knowledge, in which the definition of the requirement of an AA has been extended to include requirement for GSH status in addition to that required for protein synthesis. These results suggest that the total SAA requirement when provided as methionine only is adequate to meet the needs of the PN-fed human neonate for GSH synthesis and protein synthesis.
Although the PN-fed human neonate appears capable of synthesizing adequate cysteine for GSH synthesis, providing the total SAA as methionine only leads to increased homocysteine concentration (17,33) in the neonate. Therefore, consideration should be given to providing the total SAA as a balance between methionine and cysteine, particularly because methionine has been implicated in PN-associated cholestasis (34).
Abbreviations
- AA:
-
amino acids
- ASR:
-
absolute synthesis rate
- FSR:
-
fractional synthesis rate
- GSH:
-
glutathione
- PN:
-
parenteral nutrition
- SAA:
-
sulfur amino acids
References
Sturman JA, Gaull G, Raiha NC 1970 Absence of cystathionase in human fetal liver: is cystine essential?. Science 169: 74–76
Pascal TA, Gillam BM, Gaull GE 1972 Cystathionase: immunochemical evidence for absence from human fetal liver. Pediatr Res 6: 773–778
Gaull G, Sturman JA, Raiha NC 1972 Development of mammalian sulfur metabolism: absence of cystathionase in human fetal tissues. Pediatr Res 6: 538–547
Vina J, Vento M, Garcia-Sala F, Puertes IR, Gasco E, Sastre J, Asensi M, Pallardo FV 1995 L-cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. Am J Clin Nutr 61: 1067–1069
Pohlandt F 1974 Cystine: a semi-essential amino acid in the newborn infant. Acta Paediatr Scand 63: 801–804
Winters RW, Heird WC, Dell RB, Nicholson JF 1977 Plasma amino acids in infants receiving parenteral nutrition. In: Greene HL, Holliday MA, Munro MA (eds) Clinical Nutrition Update: Amino Acids. American Medical Association, Chicago, IL, pp 147–157
Kanaya S, Nose I, Harada T, Kai H, Ogawa M, Maki I, Tajiri H, Kimura S, Yabuuchi H, Imura K 1984 Total parenteral nutrition with a new amino acid solution for infants. J Pediatr Gastroenterol Nutr 3: 440–445
Zlotkin SH, Anderson GH 1982 The development of cystathionase activity during the first year of life. Pediatr Res 16: 65–68
Zlotkin SH, Bryan MH, Anderson GH 1981 Cysteine supplementation to cysteine-free intravenous feeding regimens in newborn infants. Am J Clin Nutr 34: 914–923
Malloy MH, Rassin DK, Richardson CJ 1984 Total parenteral nutrition in sick preterm infants: effects of cysteine supplementation with nitrogen intakes of 240 and 400 mg/kg/day. J Pediatr Gastroenterol Nutr 3: 239–244
Soghier LM, Brion LP 2006 Cysteine, cystine or N-acetylcysteine supplementation in parenterally fed neonates. Cochrane Database Syst Rev 4: CD004869
Shew SB, Keshen TH, Jahoor F, Jaksic T 2005 Assessment of cysteine synthesis in very low-birth weight neonates using a [13C6]glucose tracer. J Pediatr Surg 40: 52–56
Riedijk MA, van Beek RH, Voortman G, de Bie HM, Dassel AC, van Goudoever JB 2007 Cysteine: a conditionally essential amino acid in low-birth-weight preterm infants?. Am J Clin Nutr 86: 1120–1125
Riedijk MA, Voortman G, van Beek RH, Baartmans MG, Wafelman LS, van Goudoever JB 2008 Cyst(e)ine requirements in enterally fed very low birth weight preterm infants. Pediatrics 121: e561–e567
Zlotkin SH, Anderson GH 1982 Sulfur balances in intravenously fed infants: effects of cysteine supplementation. Am J Clin Nutr 36: 862–867
Te Braake FW, Schierbeek H, de Groof K, Vermes A, Longini M, Buonocore G, van Goudoever JB 2008 Glutathione synthesis rates after amino acid administration directly after birth in preterm infants. Am J Clin Nutr 88: 333–339
Courtney-Martin G, Chapman KP, Moore AM, Kim JH, Ball RO, Pencharz PB 2008 Total sulfur amino acid requirement and metabolism in parenterally fed postsurgical human neonates. Am J Clin Nutr 88: 115–124
Jahoor F, Wykes LJ, Reeds PJ, Henry JF, del Rosario MP, Frazer ME 1995 Protein-deficient pigs cannot maintain reduced glutathione homeostasis when subjected to the stress of inflammation. J Nutr 125: 1462–1472
Roberts SA, Ball RO, Moore AM, Filler RM, Pencharz PB 2001 The effect of graded intake of glycyl-L-tyrosine on phenylalanine and tyrosine metabolism in parenterally fed neonates with an estimation of tyrosine requirement. Pediatr Res 49: 111–119
Wilkinson DL, Bertolo RF, Brunton JA, Shoveller AK, Pencharz PB, Ball RO 2004 Arginine synthesis is regulated by dietary arginine intake in the enterally fed neonatal piglet. Am J Physiol Endocrinol Metab 287: E454–E462
Bertolo RF, Brunton JA, Pencharz PB, Ball RO 2003 Arginine, ornithine, and proline interconversion is dependent on small intestinal metabolism in neonatal pigs. Am J Physiol Endocrinol Metab 284: E915–E922
Pearson FC 1979 The Limulus amebocyte lysate endotoxin assay: current status. Am J Med Technol 45: 704–709
Zlotkin SH, Bryan MH, Anderson GH 1981 Intravenous nitrogen and energy intakes required to duplicate in utero nitrogen accretion in prematurely born human infants. J Pediatr 99: 115–120
Zlotkin SH 1984 Intravenous nitrogen intake requirements in full-term newborns undergoing surgery. Pediatrics 73: 493–496
Courtney-Martin G, Rafii M, Wykes LJ, Ball RO, Pencharz PB 2008 Methionine-adequate cysteine-free diet does not limit erythrocyte glutathione synthesis in young healthy adult men. J Nutr 138: 2172–2178
Rafii M, Elango R, Courtney-Martin G, House JD, Fisher L, Pencharz PB 2007 High-throughput and simultaneous measurement of homocysteine and cysteine in human plasma and urine by liquid chromatography-electrospray tandem mass spectrometry. Anal Biochem 371: 71–81
Paamoni-Keren O, Silberstein T, Burg A, Raz I, Mazor M, Saphier O, Weintraub AY 2007 Oxidative stress as determined by glutathione (GSH) concentrations in venous cord blood in elective cesarean delivery versus uncomplicated vaginal delivery. Arch Gynecol Obstet 276: 43–46
Reid M, Badaloo A, Forrester T, Morlese JF, Frazer M, Heird WC, Jahoor F 2000 In vivo rates of erythrocyte glutathione synthesis in children with severe protein-energy malnutrition. Am J Physiol Endocrinol Metab 278: E405–E412
Thomas B, Gruca LL, Bennett C, Parimi PS, Hanson RW, Kalhan SC 2008 Metabolism of methionine in the newborn infant: response to the parenteral and enteral administration of nutrients. Pediatr Res 64: 381–386
Jahoor F, Jackson A, Gazzard B, Philips G, Sharpstone D, Frazer ME, Heird W 1999 Erythrocyte glutathione deficiency in symptom-free HIV infection is associated with decreased synthesis rate. Am J Physiol 276: E205–E211
Malloy MH, Rassin DK 1984 Cysteine supplementation of total parenteral nutrition: the effect in beagle pups. Pediatr Res 18: 747–751
Wu PY, Edwards N, Storm MC 1986 Plasma amino acid pattern in normal term breast-fed infants. J Pediatr 109: 347–349
Shoveller AK, House JD, Brunton JA, Pencharz PB, Ball RO 2004 The balance of dietary sulfur amino acids and the route of feeding affect plasma homocysteine concentrations in neonatal piglets. J Nutr 134: 609–612
Moss RL, Haynes AL, Pastuszyn A, Glew RH 1999 Methionine infusion reproduces liver injury of parenteral nutrition cholestasis. Pediatr Res 45: 664–668
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grant number FRN 12928 from the Canadian Institutes for Health Research. Supported by scholarships from The Clinician Scientist Training Program (CSTP), The Hospital for Sick Children and The Strategic Training Institute in Health Research (STIHR), The Canadian Institute of Health Research (G.C.-M.).
Rights and permissions
About this article
Cite this article
Courtney-Martin, G., Moore, A., Ball, R. et al. The Addition of Cysteine to the Total Sulphur Amino Acid Requirement as Methionine Does Not Increase Erythrocytes Glutathione Synthesis in the Parenterally Fed Human Neonate. Pediatr Res 67, 320–324 (2010). https://doi.org/10.1203/PDR.0b013e3181ca036f
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181ca036f