Abstract
Suppressor of cytokine signaling-2 (SOCS-2) is a member of the suppressor of cytokine signaling family, implicated in the negative regulation of cytokine action through inhibition of the Janus kinase (JAK) signal transducers and activators of transcription (STAT) signal transduction pathway. We have previously reported that SOCS-2−/− mice display an increased longitudinal skeletal growth associated with a deregulated GH/IGF-I signaling. The aim of the present study was to determine the role of SOCS-2 in the regulation of bone mineral density (BMD). Dual x-ray absorptiometry (DXA) analyses demonstrated that the areal BMD of the tibia was reduced in both 4-wk-old (−8.6%) and 15-wk-old (−6.0%) SOCS 2−/− mice compared with wild-type (WT) mice. The trabecular volumetric BMD, as measured by peripheral quantitative computerized tomography (pQCT) in the metaphyseal region of the distal femur, was reduced in both 4-wk-old (−10%) and 15-wk-old (−32%) SOCS 2−/− mice compared with WT mice. pQCT analyses in the diaphyseal region of tibia also revealed that the cortical volumetric BMD was reduced in both 4-wk-old (−7%) and 15-wk-old (−3%) SOCS 2−/− mice. The cortical cross-sectional area was reduced in 4-wk-old but not in 15-wk-old SOCS 2−/− mice. In conclusion, SOCS-2 inactivation results in reduced trabecular and cortical volumetric BMD. These effects are not consistent with an augmented GH/IGF-I signaling and, therefore, the mechanism behind the reduced BMD remains to be elucidated.
Similar content being viewed by others
Main
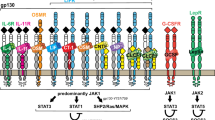
Cytokines regulate cell growth and differentiation by binding to cell surface cytokine receptors, activating signal transduction cascades, such as the JAK-STAT pathway. The SOCS are a recently discovered group of proteins that have the ability to regulate cytokine signaling. Eight members of this group, including SOCS 1–7 and CIS, have been identified (1). The SOCS transcripts are up-regulated in response to cytokine stimulation, and the corresponding SOCS proteins inhibit the cytokine signal transduction pathways, via a mechanism that involves blocking the JAK activity or STAT binding sites on cytokine receptors, creating a negative feedback loop of cytokine signaling (1). In vivo studies have revealed very diverse functions of the SOCS proteins. Hence, SOCS-1 has been demonstrated to be a regulator of lymphocyte differentiation and interferon γ signaling (2,3), whereas SOCS- 3 is believed to be essential for placental function (4). In vitro studies have shown that SOCS-2 inhibits signal transduction induced by several cytokines including IL-6, leukemia inhibitory factor (LIF), IGF-I, prolactin, and GH (1,5,6). However, only low doses of SOCS-2 inhibited GH signaling, whereas high doses were reported to, in fact, stimulate GH signaling (7). We have previously reported that mice with a targeted gene deletion of the SOCS-2 gene display increased postnatal body growth, resulting in increased long bone length and a proportionate augmentation of most visceral organs (8). These effects were associated with deregulated growth hormone/IGF-I signaling, suggesting that SOCS-2 may have an essential negative regulatory role in the GH/IGF-I signaling pathway. Both GH and IGF-I are involved in the regulation of adult bone mineral metabolism, including BMD (9). The aim of the present study was to determine the role of SOCS-2 in the regulation of BMD, and we demonstrate that SOCS-2 deficiency leads to reduced BMD in both the trabecular and the cortical bone compartment.
METHODS
Animals.
Male mice lacking SOCS-2 (SOCS-2−/−) and WT litter-mate mice were generated and genotyped as previously described (8). All mice were of a pure C57Bl/6 background, and all animal experimentation was performed in accordance with National Health and Medical Research Council of Australia guidelines (Ethics Committee approval number 2003.014). Animals were fed a diet containing 12.8 kJ/g, 0.012 mg calcium/g, 6.6% fat, 22.7% carbohydrates, and 21.7% protein.
DXA.
Measurements of aBMD of the total tibia and of a 0.3 × 0.3 mm large subregion, with a relatively high content of trabecular bone, located in the proximal metaphyseal region of tibia, were performed ex vivo with the Norland pDEXA Sabre (Norland, Fort Atkinson, WI) and the Sabre Research software (v3.6) as previously described (10). The interassay CV for these measurements was below 5%.
pQCT.
Computerized tomography (CT) was performed with the Stratec pQCT XCT Research M (Norland; v5.4B) operating at a resolution of 70 μm (11). Trabecular volumetric BMD was determined ex vivo, with a metaphyseal pQCT scan of the distal femur, and defined as the inner 45% of the total cross-sectional area. Cortical bone parameters were determined ex vivo with a mid-diaphyseal pQCT scan of the femur and tibia. The CV for these measurements was <2%.
Serum parameters.
Serum osteocalcin levels were measured using a MAb raised against human osteocalcin (Rat-MID osteocalcin ELISA, Osteometer Biotech, Herlev, Denmark). The sensitivity of the osteocalcin assay was 21.1 ng/mL and intra- and interassay CV were <10%. Serum IGF-I levels were measured by double-antibody IGF binding protein-blocked RIA (12).
Statistical analysis.
Results are presented as means ± sem. Differences in bone parameters between WT and SOCS-2 deficient mice were calculated using students t test. A p value < 0.05 was considered statistically significant.
RESULTS
Longitudinal bone growth.
The lengths of the two long bones tibia and femur were unchanged in 4-wk-old SOCS 2−/− compared with age-matched WT mice (Table 1). However, in adult mice, at 15 wk of age, SOCS-2−/− mice had both longer tibias (4.4%) and femora (5.6%) than the WT mice (Table 1).
aBMD.
aBMD of excised tibia was analyzed by DXA. The aBMD was reduced in the total tibia in both 4-wk-old (−8.6%) and in 15-wk-old (−6.0%) SOCS 2−/− compared with WT mice (Fig. 1). These reductions were more pronounced when the aBMD was analyzed in a subregion, with a relatively high content of trabecular bone, located in the proximal metaphyseal region of the tibia. In this region, the aBMD was reduced by 11.4% in 4-wk-old and by 10.7% in 15-wk-old SOCS 2−/− compared with WT mice (Fig. 1).
DXA measurements of aBMD in the total tibia (Total) and the proximal metaphyseal region (Metaphyseal; a region with a relatively high content of trabecular bone) of tibia in 4-wk-old (A) and 15-wk-old (B) WT and SOCS-2−/− mice. Values expressed as means ± sem. *p < 0.05, **p < 0.01 vs WT (n = 10 for 4-wk-old, n = 6 for 15-wk-old).
Trabecular volumetric BMD.
The results obtained from DXA measurements are a combination of effects on trabecular bone and cortical bone parameters. To be able to distinguish between effects on trabecular and cortical bone, the bones were analyzed by pQCT. The trabecular volumetric BMD was measured in the metaphyseal region of the distal femur using pQCT. Both 4-wk-old and 15-wk-old SOCS 2−/− mice displayed lower trabecular volumetric BMD (−10% and −32%, respectively) than WT mice of the same age (Fig. 2).
Cortical bone parameters.
The cortical bone mineral content (BMC) in the diaphyseal region of femur was reduced in 4-wk-old SOCS 2−/− mice compared with WT mice (Table 2). The reduction of cortical BMC was associated with a decrease in cortical volumetric BMD, cross-sectional area, and thickness (Table 2). The reduced cortical thickness was caused by a greater endosteal circumference whereas the periosteal circumference was unaffected in the 4-wk-old SOCS-2−/− mice. In adult mice, at 15 wk of age, a less severe cortical bone phenotype was seen with only the volumetric BMD significantly lower in the SOCS-2−/− mice than in the WT mice (Table 2).
Serum markers.
Serum levels of osteocalcin, a marker of bone formation/turnover, were significantly increased in 4-wk-old but not in 15-wk-old SOCS 2−/− mice compared with WT mice (Table 3). The levels of IGF-I were not significantly different between SOCS 2−/− and WT mice at any age (Table 3).
DISCUSSION
We have in the present study confirmed the finding that SOCS-2 is of importance for the regulation of skeletal growth. However, more importantly, we made the novel observations that i) SOCS-2 is a crucial regulator of BMD and ii) that this effect on BMD is not consistent with an expected augmented GH/IGF-I signaling in SOCS-2–deficient mice. GH is the most important regulator of postnatal body growth. A large part of its growth-promoting effect is mediated by GH-stimulated expression of IGF-I in the liver, but also in peripheral tissues (9,13,14). Mice with a targeted gene deletion of the SOCS-2 gene display increased postnatal body growth, resulting in increased long bone length and a proportionate augmentation of most visceral organs (8). These mice have several characteristics of deregulated GH and IGF-I signaling, such as collagen accumulation in the dermis, affected production of major urinary protein, and increased local production of IGF-I in several organs, including the heart, lungs, and spleen. These findings suggest that an important effect of SOCS-2 is to inhibit the GH/IGF-I signaling pathway, resulting in an increased growth in SOCS-2–inactivated mice (8). The high growth (hg) mouse strain displays a 30–50% increment in postnatal growth, without resulting in obesity, compared with WT mice, and has been found to carry a mutation in the SOCS-2 gene (15), further supporting the role of SOCS-2 in regulating growth.
In vitro data have revealed that SOCS-2 inhibits GH signaling at low doses, probably to a lesser extent than SOCS-1 and SOCS-3 (5). Furthermore, SOCS-2 inhibition of GH signaling has been shown effective only in low concentrations, whereas higher concentrations restore and even stimulate GH signaling, suggesting an additional role of SOCS-2 in restoring the sensitivity of inhibited GH signaling caused by other SOCS proteins (7,16). These intriguing in vitro data are supported by our recent in vivo findings, showing that both absence and over-expression of SOCS-2 cause growth enhancement in mice (8,16). The SOCS-2 over-expressing transgenic mice display enhanced growth (16), suggesting an enhanced GH signaling. Therefore, for the regulation of growth, it has been proposed that SOCS-2 has a dual effect on GH signaling. Both inactivation and over-expression of SOCS-2 result in enhanced GH signaling and growth whereas physiologic levels of SOCS-2 reduce GH signaling.
Both GH and IGF-I treatment result in an increased BMD (9,14, 17–19). Thus, the present finding of reduced BMD in SOCS-2–deficient mice is not consistent with an enhanced GH/IGF-I signaling as proposed above for the regulation of growth. One may speculate that SOCS-2 inactivation results in differential effects in the regulation of different physiologic processes, including enhanced GH signaling in the regulation of growth, while it does not affect or reduces GH/IGF-I signaling in the regulation of BMD. Alternatively, the ability of SOCS-2 to inhibit signal transduction induced by other cytokines including IL-6, LIF, and prolactin (1,20,21) might be of importance for the mechanism behind the reduced BMD in SOCS-2–deficient mice. For instance, BMD is affected in prolactin receptor–inactivated mice, and LIF has been reported to stimulate bone resorption (22,23). Gene deletion of IL-6 has been shown to prevent bone loss in mice after gonadectomy (24,25), and increased IL-6 has been found in a multitude of conditions involving increased bone resorption, such as rheumatoid arthritis and Paget's disease (26,27). Furthermore, IL-6 has been shown to directly induce bone resorption in mice calvaria (23). Hence, a reduced inhibition of IL-6 and LIF signaling in SOCS-2–deficient mice could account for increased bone resorption and resulting lower BMD in these animals. Thus, further studies are required to determine which cytokine signaling pathway is involved in the affected BMD in SOCS-2-deficient mice. The reduced BMD in SOCS 2−/− mice, seen in the present study, was demonstrated by extensive x-ray analyses, including both DXA and pQCT. DXA analysis demonstrated that the aBMD was reduced in the total tibia. Initial aBMD measurement could not distinguish whether it was an effect on the size of the bone, the trabecular volumetric BMD, the cortical volumetric BMD or a combination. Subregion analyses in the proximal metaphyseal region of the tibia, with a relatively high content of trabecular bone, indicated that the reduced aBMD, at least partly, was due to a reduced trabecular BMD in SOCS 2−/− mice. pQCT analyses were then performed to clearly distinguish between effects on trabecular and cortical bone, demonstrating that both the trabecular and cortical volumetric BMD was reduced in SOCS 2−/− mice. The cortical cross-sectional area and cortical thickness were reduced in 4-wk-old but not in 15-wk-old SOCS 2−/− mice, suggesting that the main effect on aBMD is a result of reduced trabecular and cortical volumetric BMD and to a lesser extent due to reduced size of the cortical bone.
In conclusion, SOCS-2 inactivation results in reduced trabecular and cortical volumetric BMD. These effects are not consistent with an augmented GH/IGF-I signaling, and, therefore, the mechanism behind the reduced BMD remains to be elucidated. SOCS-2 has been suggested as a possible target for the development of novel treatment strategies for children with growth disturbances (15), but the present study indicates that reduced BMD and an associated increased risk of osteoporosis is a potential side effect when the activity of SOCS-2 is affected.
Abbreviations
- aBMD:
-
areal bone mineral density
- BMD:
-
bone mineral density
- CV:
-
coefficient of variation
- DXA:
-
dual x-ray absorptiometry
- JAK:
-
Janus kinase
- pQCT:
-
peripheral quantitative computerized tomography
- SOCS:
-
suppressor of cytokine signaling
- STAT:
-
signal transducers and activators of transcription
- WT:
-
wild type
References
Krebs DL, Hilton DJ 2001 SOCS proteins: negative regulators of cytokine signaling. Stem Cells 19: 78–387
Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis D, Yoshimura A, Ihle JN 1999 SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell 98: 09–616
Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, Kay TW, Nicola NA, Hertzog PJ, Metcalf D, Hilton DJ 1999 SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98: 97–608
Roberts AW, Robb L, Rakar S, Hartley L, Cluse L, Nicola NA, Metcalf D, Hilton DJ, Alexander WS 2001 Placental defects and embryonic lethality in mice lacking suppressor of cytokine signaling 3. Proc Natl Acad Sci U S A 98: 324–9329
Ram PA, Waxman DJ 1999 SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J Biol Chem 274: 5553–35561
Zong CS, Chan J, Levy DE, Horvath C, Sadowski HB, Wang LH 2000 Mechanism of STAT3 activation by insulin-like growth factor I receptor. J Biol Chem 275: 5099–15105
Favre H, Benhamou A, Finidori J, Kelly PA, Edery M 1999 Dual effects of suppressor of cytokine signaling (SOCS-2) on growth hormone signal transduction. FEBS Lett 453: 3–66
Metcalf D, Greenhalgh CJ, Viney E, Willson TA, Starr R, Nicola NA, Hilton DJ, Alexander WS 2000 Gigantism in mice lacking suppressor of cytokine signalling-2. Nature 405: 069–1073
Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC 1998 Growth hormone and bone. Endocr Rev 19: 5–79
Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C 1999 Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(−/−) mice. J Clin Invest 104: 95–901
Vidal O, Lindberg MK, Hollberg K, Baylink DJ, Andersson G, Lubahn DB, Mohan S, Gustafsson JA, Ohlsson C 2000 Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci U S A 97: 474–5479
Blum WF, Breier BH 1994 Radioimmunoassays for IGFs and IGFBPs. Growth Regul 4: 1–19
Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson OG, Jansson JO, Ohlsson C 1999 Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A 96: 088–7092
Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D 2002 Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest 110: 71–781
Horvat S, Medrano JF 2001 Lack of Socs2 expression causes the high-growth phenotype in mice. Genomics 72: 09–212
Greenhalgh CJ, Metcalf D, Thaus AL, Corbin JE, Uren R, Morgan PO, Fabri LJ, Zhang JG, Martin HM, Willson TA, Billestrup N, Nicola NA, Baca M, Alexander WS, Hilton DJ 2002 Biological evidence that SOCS-2 can act either as an enhancer or suppressor of growth hormone signaling. J Biol Chem 277: 0181–40184
Sims NA, Clement-Lacroix P, Da Ponte F, Bouali Y, Binart N, Moriggl R, Goffin V, Coschigano K, Gaillard-Kelly M, Kopchick J, Baron R, Kelly PA 2000 Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest 106: 095–1103
Mohan S, Richman C, Guo R, Amaar Y, Donahue LR, Wergedal J, Baylink DJ 2003 Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology 144: 29–936
Sjogren K, Sheng M, Moverare S, Liu JL, Wallenius K, Tornell J, Isaksson O, Jansson JO, Mohan S, Ohlsson C 2002 Effects of liver-derived insulin-like growth factor I on bone metabolism in mice. J Bone Miner Res 17: 977–1987
Nicholson SE, Willson TA, Farley A, Starr R, Zhang JG, Baca M, Alexander WS, Metcalf D, Hilton DJ, Nicola NA 1999 Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J 18: 75–385
Pezet A, Favre H, Kelly PA, Edery M 1999 Inhibition and restoration of prolactin signal transduction by suppressors of cytokine signaling. J Biol Chem 274: 4497–24502
Clement-Lacroix P, Ormandy C, Lepescheux L, Ammann P, Damotte D, Goffin V, Bouchard B, Amling M, Gaillard-Kelly M, Binart N, Baron R, Kelly PA 1999 Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice. Endocrinology 140: 6–105
Palmqvist P, Persson E, Conaway HH, Lerner UH 2002 IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activator of NF-kappa B in mouse calvariae. J Immunol 169: 353–3362
Bellido T, Jilka RL, Boyce BF, Girasole G, Broxmeyer H, Dalrymple SA, Murray R, Manolagas SC 1995 Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J Clin Invest 95: 886–2895
Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, Ciliberto G, Rodan GA, Costantini F 1994 Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J 13: 189–1196
De Benedetti F, Massa M, Pignatti P, Albani S, Novick D, Martini A 1994 Serum soluble interleukin 6 (IL-6) receptor and IL-6/soluble IL-6 receptor complex in systemic juvenile rheumatoid arthritis. J Clin Invest 93: 114–2119
Kotake S, Sato K, Kim KJ, Takahashi N, Udagawa N, Nakamura I, Yamaguchi A, Kishimoto T, Suda T, Kashiwazaki S 1996 Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res 11: 8–95
Acknowledgements
The authors thank Anette Hansevi for excellent technical support. We also thank SWEGENE Center for Bio-Imaging, University of Göteborg, for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the Swedish Medical Research Council, the Swedish Foundation for Strategic Research, the European Commission, the Lundberg Foundation, the Torsten and Ragnar Söderberg's Foundation, the Emil and Vera Cornell Foundation, the Novo Nordisk Foundation, the Petrus and Augusta Hedlunds Foundation, the National Health and Medical Research Council, Canberra, Australia, the National Institute of Health, Bethseda, MD (grant CA-22556), the Australian Federal Government Cooperative Research Centres Program, and the Australian Research Council Postgraduate Fellowship (C.J.G).
Rights and permissions
About this article
Cite this article
Lorentzon, M., Greenhalgh, C., Mohan, S. et al. Reduced Bone Mineral Density in SOCS-2-Deficient Mice. Pediatr Res 57, 223–226 (2005). https://doi.org/10.1203/01.PDR.0000148735.21084.D3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000148735.21084.D3
This article is cited by
-
SOCS2 regulates alveolar bone loss in Aggregatibacter actinomycetemcomitans-induced periodontal disease
Inflammation Research (2023)
-
Tropomyosin-Related Kinase B (TrkB) Regulates Neurite Outgrowth via a Novel Interaction with Suppressor of Cytokine Signalling 2 (SOCS2)
Molecular Neurobiology (2019)
-
Genome-wide patterns of copy number variation in the diversified chicken genomes using next-generation sequencing
BMC Genomics (2014)
-
Lack of association between suppressor of cytokine signaling-3 gene polymorphism and susceptibility and curve severity of adolescent idiopathic scoliosis
European Spine Journal (2014)
-
Inflammation and linear bone growth: the inhibitory role of SOCS2 on GH/IGF-1 signaling
Pediatric Nephrology (2013)