Abstract
In fetal lambs, severe hypoxia (SH) will lead to brain damage. Mild hypoxia (MH) is thought to be relatively safe for the fetal brain because compensating mechanisms are activated. We questioned whether MH, leading to mild acidosis, induces changes in cerebral metabolism. Metabolites in cerebrospinal fluid (CSF) samples, as analyzed by proton magnetic resonance spectroscopy, were studied in two groups of seven anesthetized near-term fetal lambs. In group I, SH leading to acidosis with an arterial pH <7.1 was achieved. In group II, MH with an intended pH of 7.23–7.27 was reached [start of MH (SMH)], and maintained during 2 h [end of MH (EMH)]. During SH, choline levels in CSF, a possible indicator of cell membrane damage, were increased. Both during SH and at EMH, CSF levels of lactic acid, alanine, phenylalanine, tyrosine, lysine, branched chain amino acids, and hypoxanthine were increased compared with control values and with SMH, respectively. At EMH, the hypoxanthine CSF-to-blood ratio was increased as compared with SMH. These results indicate that prolonged MH leads to energy degradation in the fetal lamb brain and may not be as safe as assumed.
Similar content being viewed by others
Main
Perinatal asphyxia may result in brain damage with subsequent motor and cognitive deficits(1–2). The development of neuropathological lesions depends on the degree and duration of the impaired cerebral oxygen supply. Fetal capillary blood sampling during labor enables the measurement of pH. On the basis of such a pH value, a decision is made to intervene or to continue labor. It is clear that after SH, which results in an arterial pH <7.00, fetal brain damage may occur(3). Whether MH will lead to neurologic impairment is not well established. This leads to a therapeutic dilemma if such a situation occurs during labor.
Experimental animal studies have established the effects of SH. When impaired oxygen supply leads to hypotension, histologic brain damage has been described(4–5). Few studies(6–8) have focused on the effect of MH on the brain, and results are somewhat conflicting. In most of these studies no histologic damage is found, although some authors described white matter injury(6–7) and delayed neuronal migration(7–8). However, MH might lead to an impairment of fetal brain metabolism that cannot be confirmed in histologic preparations.
1H-NMR spectroscopy is a powerful technique that makes it possible to study various metabolites simultaneously. When neuronal cell damage has occurred, metabolites are released into the cerebrospinal fluid (CSF)(9). With in vitro 1H-NMR spectroscopy, it is possible to measure many CSF metabolites including lactic acid and purines. The purines hypoxanthine and xanthine are produced when ATP is degraded as a result of oxygen shortage. High concentrations of hypoxanthine and xanthine in CSF of human neonates have been associated with later evidence of brain damage or subsequent death(10).
The present study investigates the effect of MH with subsequent mild acidosis on fetal brain metabolic responses in fetal lambs. We measured metabolites in CSF and blood, which are directly associated with the energy metabolism of the cell and metabolites in CSF related to cell death during N, MH, and prolonged MH. We hypothesized that, at the onset of a period of MH, no or minimal changes in CSF metabolites are present, and we questioned whether changes in CSF metabolites will be present if the MH is maintained for 2 h.
METHODS
Animal preparation
After 48 h fasting, a total of 23 pregnant ewes of the Dutch Texel breed were operated on at a gestational age of 127–132 d (term is 147 d). General anesthesia was induced with 25 mg/kg pentobarbital with 0.5 mg atropine i.v. and was maintained with 2–3% enflurane in a 2:1 mixture of nitrous oxide and oxygen. The temperature of the ewe was kept constant by means of a thermostatic heating pad underneath the animal. A paramedian abdominal incision was made followed by hysterotomy. The fetal head was extracted and a polyvinyl sample catheter (outer diameter 1.6 mm, inner diameter 0.8 mm) was inserted via the left axillary artery for sampling of arterial blood.
Around the left common carotid artery an animal perivascular ultrasonic blood flow transducer was placed (2SB763, Transonic System, New York, NY, U.S.A.). Transonic gel was used to optimize contact of the transducer with the carotid artery.
To reach the sagittal sinus, the scalp was incised and the periosteum spread aside. A 14-G Venflon needle was used to make a hole through the bone over the sagittal sinus at the place where the lamboid suture intersects the sagittal sinus. A polyvinyl catheter (outer diameter 1.6 mm, inner diameter 0.8 mm) was then placed in the sagittal sinus. A rubber disc was fixed at the end of the catheter and was glued to the skull. Arterial blood from the axillary artery and venous blood from the sagittal sinus were drawn simultaneously.
For the CSF sampling, a midline incision was made in the skin of the dorsal surface of the fetal neck. Entrance to the cisterna magna was accomplished with a 14-G Venflon needle and CSF of the spinal cavity was collected with a 1-mL syringe.
The experiments were approved by the local ethical committee for animal research.
Measurements
Fetal blood samples were analyzed within 2 min to assess Sao2, pH, Hb, and blood gases (ABL 510, Radiometer, Copenhagen, Denmark). Cao2 and BEecf were calculated according to the formula used by the blood gas analyzer. Fetal blood for glucose and lactic acid measurements were collected in special syringes, stored on ice, and measured within 1.5 h. Arterial lactic acid was measured in whole blood by a spectrophotometric method (Cobas Mira Plus analyzer, Roche Diagnostics Systems, Almere, The Netherlands). Glucose was measured in whole blood with an enzymatic colorimetric test (SYS 3 BM/Hitachi 747, Boehringer, Mannheim, Germany). Levels of hypoxanthine, xanthine, and inosine were determined in plasma using an HPLC procedure with a reversed-phase column (Alltima C18, Alltech Associates, Deerfield, IL, U.S.A.) and a Millennium32 data system (Millipore-Waters, Marlbourough, MA, U.S.A.), after centrifugation of the blood at 3000 revolutions/min for 10 min and freezing to −80°C. FBP was determined with an open-tip catheter in the left axillary artery using disposable transducers. FHR was derived from the pulsating FBP signal. The continuous signals, together with the continuous flow signal of the left carotid artery, were stored on a personal computer using a data acquisition program (Poly Physiologic Analysis Package, Inspektor Research System, Amsterdam, The Netherlands).
The CSF samples (0.8–1.2 mL) were immediately centrifuged (10 min at 3000 revolutions/min) to eliminate erythrocytes, if present, and stored at −80°C. Pretreatment of the CSF samples for nuclear magnetic resonance (NMR) measurements has been described by Wevers et al.(11). All samples were deproteinized.
NMR measurements
1H-NMR spectroscopy of CSF samples was performed on a 600-MHz Bruker spectrometer (Bruker Analytische Messtechnik, Karlsruhe, Germany) as described previously(11). The temperature during the measurements was 25°C, and NMR tubes with a diameter of 5 mm were used. CSF sample volume varied between 130 and 500 μL. The sample volume was filled up to 500 μL with water. For the 1H-NMR measurements, a 60° radio frequency pulse was used. The delay between two successive pulses was 6 s. The number of scans for each experiment was 128. The water resonance was presaturated during the relaxation delay. The FID was recorded in 32,768 data points with a sweep width of 7184 Hz.
NMR data analysis
A sine-bell squared filter was used. The FID were Fourier transformed after the FID was zero filled to 65,536 data points. The chemical shift of the internal standard TSP was set at a position of 0 ppm. For analysis of the NMR spectra, we used 1D WinNMR and Winfit software (Bruker Analytische Messtechnik). The phase and baseline of the spectra were corrected manually. Resonances in the spectra were fitted semi-automatically to a Lorentzian lineshape model function in the frequency domain. Quantitative data were obtained by calculating metabolite concentrations from the area of its corresponding resonance(s) with respect to the area of the internal standard TSP. When it was impossible to fit the resonances of a metabolite properly because of overlapping resonances, the peak height of the resonances was compared with the peak height of TSP (100%) (expressed as %TSP in the tables).
The pH of the CSF influences the chemical shift of some metabolites. Therefore, the pH of the samples was standardized (pH 2.50 ± 0.05).
Experimental procedures
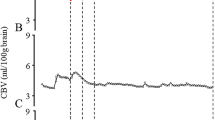
After surgery and recovery (arterial pH ≥7.35), baseline values were obtained during a control period of 1 h. Fetal acid-base balance and blood gases, drawn from the axillary artery and sagittal sinus, were determined every 15 min during baseline and every 5–10 min during the hypoxic period. Fetal hypoxia was achieved by decreasing the maternal Fio2. Figure 1 shows examples of the experimental protocol in the two groups of fetal lambs. In group I (SH), CSF was sampled at the end of the baseline and at the end of the hypoxic period. In group II (MH), the CSF sample was taken when pH fell to between 7.23 and 7.27 (SMH). In this mild hypoxic group, the pH was kept between 7.23 and 7.27 for 2 h. This was achieved by varying the maternal Fio2 based on repeated fetal blood sampling. At the end of the 2 h, CSF was sampled again (EMH).
Data analysis and statistics
Oxygen extraction was calculated by dividing Cavo2 by Cao2. Measured and calculated fetal blood variables, arterial blood values, and fetal CSF values were analyzed by paired t test in group I. Data in group II were analyzed with a linear mixed model for three samples. When there was a significant influence of time, the differences between control and SMH and between SMH and EMH were tested. The procedure “MIXED” of the SAS statistical package (SAS, Cary, NC, U.S.A.) was used. For the CSF metabolites that were not detectable with 1H-NMR, half of the detection level of the metabolite was taken as value. P Values ≤ 0.05 were considered significant. Values are expressed as mean ± SD. Median (range) is given for the CSF metabolites.
RESULTS
In group I, 12 fetal lambs were operated on. Two consecutive CSF samples in the same animal could be obtained in seven fetal lambs. In group II, 11 fetal lambs were operated on; baseline values were not satisfactory (pH <7.35) in 4 lambs and these fetal lambs were excluded. In the other seven fetal lambs, at least two CSF samples per animal could be obtained. In three of these seven fetal lambs, three consecutive CSF samples could be collected in the same animal; in two of the fetal lambs the sample at SMH was not obtained; and in the two remaining lambs no CSF could be obtained at EMH.
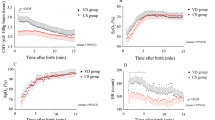
Figure 1 gives a representative example of the course of an experiment with a fetal lamb from group I and a fetal lamb from group II. Measured and calculated variables are shown in Table 1. During SH (Table 1, group I), the Sao2 decreased from 65.8 ± 15.7% to 10.1 ± 4.1%. The hypoxic period varied from 85 to 150 min. Table 1 (group II) shows the same variables during N, SMH, and EMH; Sao2 decreased to values between 28.2 ± 11.4% and 39.6 ± 13.8%. Acidosis developed with a mean pH of 7.07 ± 0.04 at the end of the severe hypoxic period in group I, which was associated with a BEecf of −12.5 ± 1.4 mM. During MH (group II), a pH of 7.25 ± 0.04 was reached. Pco2 and Hb concentrations of the fetal arterial blood increased significantly during SH and did not change during MH. FBP fell from 45 ± 5 to 26 ± 7 mm Hg during SH and did not change during MH. FHR decreased during SH and did not change during MH. In group I, the blood flow of the left carotid artery almost doubled to a maximum value of 70 ± 15 mL/min, which was reached 11–139 min after the start of hypoxia, between 12% and 71% of the total time of the hypoxic period. Hereafter, the flow collapsed due to ongoing SH. During MH, the carotid blood flow did not change significantly. Cavo2, cerebral oxygen extraction, and cerebral oxygen consumption of the fetus decreased at the end of the SH period compared with control values. During SMH, the Cavo2 and cerebral oxygen consumption decreased in comparison with control levels, and did not change further during EMH. Cerebral oxygen extraction remained constant during the experiment in group II.
Figure 2 shows parts of 1H-NMR spectra of CSF samples obtained from one fetal lamb during N and SH.
Fetal CSF levels are shown in Table 2. In group I, CSF levels of lactic acid, hypoxanthine, alanine, phenylalanine, tyrosine, lysine, BCAA, β-hydroxy-butyric acid, and choline were increased at the end of the period of SH, whereas glucose decreased. In group II, CSF lactic acid increased at SMH compared with control values, and increased to a higher level at EMH. CSF hypoxanthine, alanine, phenylalanine, tyrosine, lysine, and BCAA were increased at EMH compared with SMH. There were no statistical differences between control values of group I and II (unpaired t test).
Table 3 shows the arterial blood concentrations of glucose, lactic acid, hypoxanthine, xanthine, and inosine. In group I, arterial concentrations of lactic acid, hypoxanthine, xanthine, and inosine increased, whereas glucose decreased. In group II, arterial lactic acid increased during SMH in comparison with control values, and was not changed after 2 h of MH compared with SMH. Arterial hypoxanthine and inosine were elevated at SMH, whereas both hypoxanthine and inosine decreased to control levels after 2 h of MH.
Table 3 also shows the CSF-to-blood ratio of glucose, lactic acid, hypoxanthine, xanthine, and inosine concentrations. During SH, the CSF-to-blood ratio of glucose decreased, and the CSF-to-blood ratio of lactic acid increased. At EMH, the CSF-to-blood ratio of hypoxanthine was increased in comparison with SMH.
DISCUSSION
Perinatal asphyxia and its sequelae such as cerebral palsy are extensively investigated. Cerebral damage occurs in animal studies when the acidosis is severe (pH <7.0), at which time systemic hypotension may be present(3). Hypotension is considered to be the main cause of cerebral damage(4). Wagner et al.(4) found that the brains of mid-gestational sheep fetuses remained intact after hypoxia if FBP was maintained above 30 mm Hg. MH leads to activation of compensatory mechanisms such as redistribution of blood flow, and it is expected that decompensation of cerebral metabolism leading to cerebral damage will not occur. However, prolonged MH may lead to changes in cerebral metabolism, which may be responsible for minor neurologic deficits.
In group I (SH), FBP dropped below this limit of 30 mm Hg, pH decreased to 7.07, and cerebral oxygen consumption was decreased at the end of the hypoxic period. In this group, the above-cited criteria for cerebral damage were met. The elevated choline in the CSF during SH (group I) may indicate cerebral membrane damage, as choline is a precursor of phospholipids, a component of cell membranes. Studies in rats showed an increase in cerebral extracellular choline concentration after ischemia or hypoxia(12). In group II (MH), FBP did not fall below 30 mm Hg and pH decreased moderately to 7.25. The cerebral oxygen consumption was only slightly reduced at SMH and did not differ significantly from control levels at EMH. Cerebral O2 extraction and carotid blood flow remained stable during the 2 h of MH. In this group, none of the criteria for cerebral damage were met. Choline, as a possible indicator of cell membrane damage, was not elevated in CSF. However, several other metabolites associated with cerebral damage, such as lactic acid and hypoxanthine, were increased in the CSF both at EMH and during SH compared with SMH and control values, respectively.
Lactic acid
During SH, CSF lactic acid reached pathologic levels of 10 mM and higher, which is associated with low Apgar scores in asphyxiated neonates(13). During the period of MH, the concentrations of lactic acid increased in CSF in comparison with control values, indicating increased cerebral anaerobic metabolism. However, pathologic CSF lactic acid levels of 10 mM were not reached. It is likely that oxidative metabolism is partly compromised and metabolic acidosis starts.
Purines
The CSF hypoxanthine levels in the fetal lambs during SH and MH were in the same range as the CSF hypoxanthine concentrations in neonates who developed brain damage or who died(10). Studies of fetal CSF metabolites are sparse, however, de Haan et al.(14) measured CSF purines (including hypoxanthine) and lactic acid during hypoxia in unanesthetized fetal lambs. During SH, CSF lactic acid and hypoxanthine rose to higher (3.5 and 5 times, respectively) levels in comparison with the values of the study of de Haan et al.(14). Although in both studies the same level of metabolic acidosis was reached, hypotension was not present in the fetal lambs of the study of de Haan et al.(14), which may explain the higher CSF concentrations of lactic acid and hypoxanthine in the present study.
Hypoglycemia is another factor that may play a role in the cause of the high levels of CSF and blood hypoxanthine in the present study during SH. However, cerebral glucose and oxygen uptake remained unchanged in hypoglycemic fetal lambs after maternal fasting of 36 h(15). During MH, the glucose levels in CSF and blood did not change compared with control values in the fetal lambs. The high levels of CSF and blood hypoxanthine at EMH are therefore caused by the prolonged hypoxia.
Extracellular levels of hypoxanthine reflect the breakdown of intracellular nucleotides, and are therefore indicative for cellular energy shortage(9). The net loss of hypoxanthine from the brain in fetal lambs occurs in combination with severe asphyxia(16). The hypoxanthine concentration in CSF and the hypoxanthine CSF-to-blood ratio were increased after 2 h of MH compared with the SMH period. The duration of MH in this study may be long enough to cause degradation of cerebral ATP. Mild hypoxic conditions resulting in an acidosis with a pH of 7.25, which is called “still normal” in the human fetus(17), may become harmful when the duration of the hypoxic event is long enough.
Alternative substrates for energy production
If hypoxia results in energy depletion, the neonatal brain has the capability of using several alternative substrates for energy production such as the ketone body β-hydroxy-butyric acid. Brains of human fetuses readily metabolize β-hydroxy-butyric acid(18). Studies in animals and humans have suggested that amino acids are important substrates for fetal energy and growth requirements(19). Perinatal brain also metabolizes BCAA(20–21). In the present study, CSF β-hydroxy-butyric acid increased during SH. During prolonged SH, BCAA, alanine, phenylalanine, tyrosine, and lysine concentrations in CSF were elevated to similar levels. Elevated levels of BCAA(22–23), alanine, phenylalanine, and tyrosine(23) were found in the CSF of asphyxiated infants. The elevated BCAA and β-hydroxy-butyric acid levels may suggest mobilization of energy substrates from their stores. During MH, β-hydroxy-butyric acid was not elevated in CSF. Apparently, it was not necessary to mobilize this energy substrate, in contrast to the group with SH. Free amino acids in CSF may be due to the degeneration of nerve cells injured by hypoxia.
Role of the BBB
The CSF levels of the metabolites are dependent on the integrity of the BBB. Under normoxic conditions, the BBB of the fetal sheep seems to be relatively impermeable to lactic acid(24) and impermeable to hypoxanthine. The BBB may be particularly susceptible to the effects of asphyxia during development. During SH, a disruption of the BBB might be expected(25). Damage to the BBB during SH might also be an explanation for the increase in the CSF levels of hypoxanthine and lactic acid. Lactic acid is able to cross the BBB of near-term fetal lambs during asphyxia(16). During SH, CSF lactic acid and hypoxanthine levels may therefore present serum levels instead of being indicative of cerebral anaerobic metabolism. However, after MH it is unlikely that the BBB becomes permeable. CSF lactic acid and hypoxanthine levels during MH therefore represent cerebral metabolism.
Anesthesia
Anesthetics may have a protective effect on the brain. Of all anesthetics, inhalation anesthetics have the least influence on cerebral metabolism. During inhalation anesthesia with isoflurane or halothane, the balance of cerebral oxygen supply-to-demand is better maintained in asphyxiated fetal lambs(26).
In conclusion, during SH, the criteria of cerebral derangement were met and metabolites of cerebral energy degradation and neuronal cell damage were measured in CSF. After prolonged MH, cerebral energy is degraded. Compensating mechanisms seemed not to be able to maintain oxidative metabolism in the fetal brain. Whether this may result in persisting minor motor and cognitive deficits is not known. If there is indeed a safety margin, it does not depend on the state of oxygenation alone but also, to a similar extent, on the duration of this MH.
Abbreviations
- BBB:
-
blood-brain barrier
- BCAA:
-
branched chain amino acids
- BEecf:
-
extracellular fluid base excess
- bpm:
-
beats per minute
- carotid BF:
-
carotid blood flow
- Cao2:
-
arterial oxygen content
- Cvo2:
-
venous oxygen content
- Cavo2:
-
arteriovenous difference in oxygen content
- EAA:
-
excitatory amino acids
- EMH:
-
end of mild hypoxia
- FBP:
-
fetal blood pressure
- FHR:
-
fetal heart rate
- FID:
-
free induction decay
- Fio2:
-
fraction of oxygen in inspiratory gas
- 1H-NMR:
-
proton nuclear magnetic resonance
- MH:
-
mild hypoxia
- N:
-
normoxia
- nd:
-
not detectable
- Pco2:
-
carbon dioxide tension
- ppm:
-
parts per million
- SH:
-
severe hypoxia
- Sao2:
-
arterial oxygen saturation
- Svo2:
-
venous oxygen saturation
- SMH:
-
start of mild hypoxia
- TSP:
-
trimethylsilyl-2,2,3,3,-tetradeuteropropionic acid
REFERENCES
Low JA, Galbraith RS, Muir DW, Killen HL, Pater EA, Karchman EJ 1988 Motor and cognitive deficits after intrapartum asphyxia in the mature fetus. Am J Obstet Gynecol 158: 356–361
Goodwin TM, Belai I, Hernandez P, Durand M, Paul RH 1992 Asphyxial complications in the term newborn with severe umbilical acidemia. Am J Obstet Gynecol 167: 1506–1512
Low JA 1993 The relationship of asphyxia in the mature fetus to long-term neurologic function. Clin Obstet Gynecol 36: 82–90
Wagner KR, Ting P, Westfall MV, Yamaguchi S, Bacher JD, Meyers RE 1986 Brain metabolic correlates of hypoxic-ischemic cerebral necrosis in mid-gestational sheep fetuses: significance of hypotension. J Cereb Blood Flow Metab 6: 425–434
Tan WKM, Williams CE, During MJ, Mallard CE, Gunning MI, Gunn AJ, Gluckmann PD 1996 Accumulation of cytotoxins during the development of seizures and edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatr Res 39: 791–797
Penning DH, Grafe MR, Hammond R, Matsuda Y, Patrick J, Richardson B 1994 Neuropathology of the near-term and midgestation ovine fetal brain after sustained in utero hypoxemia. Am J Obstet Gynecol 170: 1425–1432
Rees S, Stringer M, Just Y, Hooper SB, Harding R 1997 The vulnerability of the fetal sheep brain to hypoxemia at mid-gestation. Dev Brain Res 103: 103–118
Braaksma MA, Douma BR, Nyakas C, Luiten PG, Aarnoudse JG 1999 Delayed neuronal migration of protein kinase C gamma immunoreactive cells in hippocampal CA1 area after 48 h of moderate hypoxemia in near term ovine fetus. Dev Brain Res 144: 253–260
Harkness RA 1988 Hypoxanthine, xanthine and uridine in body fluids, indicators of ATP depletion. J Chromatogr 429: 255–278
Harkness RA, Lund RJ 1983 Cerebrospinal fluid concentrations of hypoxanthine, xanthine, uridine and inosine: high concentrations of the ATP metabolite, hypoxanthine, after hypoxia. J Clin Pathol 36: 1–8
Wevers RA, Engelke U, Wendel U, de Jong JGN, Gabreëls FJM, Heerschap A 1995 Standardized method for high-resolution 1H-NMR spectroscopy of cerebrospinal fluid. Clin Chem 41: 1–8
Scremin OU, Jenden DJ 1989 Focal ischemia enhances choline output and decreases acetylcholine output from rat cerebral cortex. Stroke 20: 92–95
Mathew OP, Bland H, Boxerman SB, James E 1980 CSF lactate levels in high risk neonates with and without asphyxia. Pediatrics 66: 224–227
de Haan HH, IJzermans ACM, de Haan J, Van Belle H, Hasaart THM 1994 Effects of surgery and asphyxia on levels of nucleosides, purine bases, and lactate in cerebrospinal fluid of fetal lambs. Pediatr Res 36: 595–600
Richardson BS, Hohimer AR, Bissonnette JM, Machida CM 1983 Cerebral metabolism in hypoglycemic and hyperglycemic fetal lambs. Am J Physiol 245: R730–R736
Thiringer K, Blomstrand S, Hrbek A, Karlsson K, Kjellmer I 1982 Cerebral arterio-venous difference for hypoxanthine and lactate during graded asphyxia in the fetal lamb. Brain Res 239: 107–117
Saling E 1964 Die Blutgasverhältnisse und der Säure-Basen-Haushalt des Feten bei ungestörtem Geburtsablauf. Zeitschrift für Geburtshilfe und Gynäkologie 161: 262–292
Adam PAJ, Räihä N, Rahiala EL, Kekomäki M 1975 Oxidation of glucose and D-B-OH-butyrate by the early human fetal brain. Acta Paediatr Scand 64: 17–24
Lemons JA, Adcock EW III, Jones MD, Naughton MA, Meschia G, Battaglia FC 1976 Umbilical uptake of amino acids in the unstressed fetal lamb. J Clin Invest 58: 1428–1434
Vahvelainen M, Oja S 1972 Kinetics of influx of phenylalanine, tyrosine, tryptophan, histidine and leucine into slices of brain cortex from adult and 7-day-old rats. Brain Res 40: 477–485
Warshaw JB, Terry ML 1976 Cellular energy metabolism during fetal development. VI. Fatty acid oxidation by developing brain. Dev Biol 52: 161–166
Kanedo K 1985 The effect of perinatal anoxia on amino acid metabolism in the developing brain. Part II: the effect of perinatal anoxia on the free amino acid patterns in CSF of infants and children. Brain Dev 7: 400–407
Hagberg H, Thornberg E, Blennow M, Kjellmer I, Lagercrantz H, Thiringer K, Hamberger A, Sandberg M 1993 Excitatory amino acids in the cerebrospinal fluid of asphyxiated infants: relationship to hypoxic-ischemic encephalopathy. Acta Paediatr 82: 925–929
Turbow RM, Curran-Everett D, Hay WW, Jones DJ 1995 Cerebral lactate metabolism in near-term fetal sheep. Am J Physiol 269: R938–R942
Lou HC, Lassen NA, Tweed WA, Johnson G, Jones M, Palahniuk RJ 1979 Pressure-passive cerebral blood flow and breakdown of the blood-brain barrier in experimental fetal asphyxia. Acta Paediatr Scand 68: 35–44
Baker BW, Hughes SC, Schnider SM, Field DR, Rosen MA 1990 Maternal anesthesia and the stressed fetus: effects of isoflurane on the asphyxiated fetal lamb. Anesthesiology 72: 65–70
Acknowledgements
The authors thank Theo Arts and Alex Hanssen of the Central Animal Laboratory Nijmegen and Sjaak van Asten for their assistance. 1H-NMR spectra were recorded at the Dutch hf-NMR facility at the Department of Biophysical Chemistry, University of Nijmegen, The Netherlands (department head, Prof. C.W. Hilbers). We thank J. Joordens for invaluable help and assistance. Furthermore, we gratefully acknowledge the help of Dr. R.A. de Abreu, K. Wethly and A. Stegeman of the Laboratory for Pediatrics and Neurology for the measurement of the blood samples for purines and pyrimidines. We thank Henk van Lier for performing the statistical analyses with the linear mixed model.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Nellcor Puritan Bennett Inc., Pleasanton, CA, U.S.A.
Rights and permissions
About this article
Cite this article
Van Cappellen Van Walsum, AM., Jongsma, H., Wevers, R. et al. Hypoxia in Fetal Lambs: A Study with 1H-NMR Spectroscopy of Cerebrospinal Fluid. Pediatr Res 49, 698–704 (2001). https://doi.org/10.1203/00006450-200105000-00015
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200105000-00015