Abstract
Necrotizing enterocolitis (NEC) causes approximately 4000 deaths/y and significant morbidity among U.S.-born preterm infants alone. Various combinations of inadequate tissue oxygenation, bacterial overgrowth, and enteral feeding with immaturity may cause the initial damage to intestinal mucosa that culminates in necrosis. Presently, there is not a way to predict the onset of the disease or to prevent its occurrence. As part of risk-benefit assessment, we compared disease in hospitalized preterm infants fed a commercial (control) preterm formula or an experimental formula with egg phospholipids for a randomized, double-masked, clinical study of diet and infant neurodevelopment. Infants fed the experimental formula developed significantly less stage II and III NEC compared with infants fed the control formula (2.9 versus 17.6%, p < 0.05), but had similar rates of bronchopulmonary dysplasia (23.4 versus 23.5%), septicemia (26 versus 31%), and retinopathy of prematurity (38 versus 40%). Compared with the control formula, the experimental formula provided 7-fold more esterified choline, arachidonic acid (AA, 0.4% of total fatty acids), and docosahexaenoic acid (0.13%). Phospholipids are constituents of mucosal membranes and intestinal surfactant, and their components, AA and choline, are substrates for intestinal vasodilatory and cytoprotective eicosanoids (AA) and the vasodilatory neurotransmitter, acetylcholine (choline), respectively. One or more of these components of egg phospholipids may have enhanced one or more immature intestinal functions to lower the incidence of NEC in this study. Regardless of the potential mechanism, a larger randomized trial designed to test the effect of this egg phospholipid-containing formula on NEC seems warranted.
Similar content being viewed by others
Main
Hospitalized preterm infants are watched closely for signs of feeding intolerance, which can signal the onset of NEC or infection. These conditions cause significant mortality and morbidity in this population. NEC occurs in approximately 10% of infants of 1500 g birth weight (1) and has been estimated to be responsible for approximately 4000 deaths/y in the United States alone (2). The long-term growth and development of preterm infants who survive NEC may be compromised by prolonged delays in enteral feeding (3). Even preterm infants who do not develop NEC may have poor nutrient intake and suboptimal growth and development, if enteral feeding is delayed or provided by formulas too low in nutrients for fear of NEC.
Typically, NEC occurs suddenly and without warning in infants who have tolerated enteral feeding for 2-6 wk. NEC is associated with many conditions that reduce mesenteric blood flow. These include immaturity, polycythemia, intrauterine growth retardation, asphyxia, exposure to cocaine, respiratory distress syndrome, exchange transfusions, intravascular catheter placement, proinflammatory cytokines, cold stress, fluid overload, hyperosmolar solutions, abdominal distention, and portal hypertension (1,4–10). Poor mesenteric blood flow may lead to intestinal hypoxia and injury with decreased mucosal surface area and loss of barrier function (11,12). Overgrowth of atypical organisms, enteral feedings, and inflammatory cytokines may cause additional injury to the intestinal mucosa (13,14).
NEC has been reported to decrease with antenatal steroids (15,16), human milk feeding (17), low pH formula (18), enteral IgA (19), and antibiotics (20,21). The putative mechanisms include enhanced barrier function (antenatal steroids) (22), immunoprotection (human milk feeding, IgA) (23–25), cell growth (human milk) (26), and bacteriostasis (antibiotics, low pH formula) (18,20,21). None of these interventions has eliminated NEC, and each is associated with some problem such as poor availability, metabolic acidosis (18), antibiotic resistance (27,28), or the inability to delay preterm birth. The addition of egg phospholipids to formula feedings produced a near-total reduction in NEC in a nursery population of very low birth weight infants historically at high risk for the disease (1).
METHODS
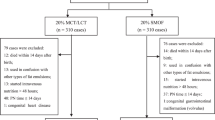
Subject selection. Study infants were enrolled continuously between September 1992 and March 1997, under a protocol approved by The University of Tennessee, Memphis, Institutional Review Board, and infants received care in the Newborn Center of the same institution. Infants ≤32 wk of gestation (29) weighing 725-1375 g, and in the ≥5th percentile for weight (30) at birth whose mothers chose formula feeding were eligible for enrollment. No infant received human milk.
Other exclusion criteria were periventricular/ intraventricular hemorrhage > grade 2, cardiac, renal or hepatic dysfunction, maternal history of alcohol or drug abuse, congenital malformations, sepsis at birth, and pulmonary disease that did not improve over the first days of life. Except for one infant who developed an intraventricular hemorrhage, infants were removed after enrollment only if enteral feeding was discontinued for >7 cumulative days after full enteral feeding (100 kcal or 416 kJ · kg body weight-1 · d-1). The exclusion and removal criteria were included to minimize confounding of the main study outcomes of first year growth and development that will be reported when the postdischarge portion of the study is complete. A total of 120 infants were randomized to diet, but one infant was transferred after 7 d because his insurance company required that his care be given in another hospital. All remaining 119 randomized infants were included in the in-hospital evaluation reported here.
Experimental design. The study was a randomized, double-masked clinical trial with infants assigned to one of three feeding regimens. The main purpose of the study was to look at the effects of providing preterm infants with a diet containing AA and DHA in amounts typically reported for milk of American women (31) from birth or after discharge home. However, diseases occurring after assignment to diet were prospectively monitored as part of risk benefit assessment of the diets. Two regimens received a commercial preterm formula (Similac Special Care) during their nursery days. They constituted the control group in this report. One of these two regimens changed to experimental formula after discharge from the hospital, the other continued to receive control formula. The group assigned to the third regimen, and that constituted the experimental group in this report, was fed experimental formula in the hospital and after discharge home. Both the control and experimental formulas were masked to avoid identification. Only the lipid blends of the two formulas differed (Table 1), resulting in different amounts of formula total phospholipids (Table 1), fatty acids (Table 2), and choline (Table 3). The randomization to diet was successful as illustrated in Table 4, which shows the neonatal and perinatal characteristics of the groups. The formulas were manufactured by Ross Products Division of Abbott Laboratories. Infants were fed Similac Special Care under its commercial label until randomized to their treatment regimen at a mean of 4.9 d of age. All infants were fed by nasogastric infusion (6 h on and 2 h off) until they weighted 1.5 kg, then at 3-h intervals by nipple or bolus infusion until hospital discharge.
Eligible infants were stratified in three birth weight categories (725-925, 926-1150, and 1151-1375 g) with equal numbers of male and female infants. Gender was unmasked to permit enrollment of eligible infants regardless of gender. To guarantee that the groups remained balanced for the planned postdischarge outcomes, infants who were lost from the study were replaced by the next infant who met the same gender and weight criteria. Enrollment continued until 30 infants per regimen could be followed through 4 mo of corrected age.
In-hospital monitoring and diagnoses. All 119 infants (115 singletons, 4 twins) were evaluated for diseases occurring during their hospitalization regardless of their availability or eligibility for the postdischarge phase of the study. Infants were weighed, and total energy intake was recorded daily while in the hospital. Starting with the first study day, the energy per kg body weight from parenteral and enteral sources was summed and averaged for each subsequent complete 7-d interval. Weekly weight gains were calculated for these same 7-d intervals. Only infants remaining in the study for the entire week were included in the calculations of weekly energy intake and growth reported here, because the purpose of including these data was to compare nutritional support before the onset of NEC. Hours of total oxygen supplementation and mechanical ventilation were recorded.
Infants with feeding intolerance, gastrointestinal bleeding, dysmotility, abnormal bowel sounds, abdominal distention or tenderness, or bilious emesis were evaluated for NEC. Diagnosis of NEC stage II or III was made by radiographic observations, including intestinal pneumatosis, portal venous air, and peritoneal free air. Modified (32) Bell's criteria for staging of NEC (33) were used to distinguish NEC stages. Two neonatologists confirmed the diagnosis of NEC. All infants diagnosed with NEC were noted to have elevated C-reactive protein (>0.9 mg/dL), added evidence of ongoing disease.
Septicemia, bronchopulmonary dysplasia, and retinopathy of prematurity were other disease that occurred after randomization. Sepsis was confirmed by positive blood cultures and elevated C-reactive protein (34). The diagnosis of bronchopulmonary dysplasia was dependent upon a need for supplemental oxygen on d 28 of life and radiographic changes as described by Northway et al. (35). Retinopathy of prematurity was graded by standardized criteria (36) and categorized according to the more severely affected eye (Table 5).
Plasma phospholipid concentration and fatty acid composition. Plasma PC and PE AA and DHA concentrations were determined after extracting total plasma lipids, separating the individual phospholipids by thin layer chromatography, transesterifiying phospholipid fatty acids with boron trifluoride-methanol to yield fatty acid methyl esters, and separating and quantifying the individual fatty acids by gas liquid chromatography on a 0.25 mm × 30-m fused silica column with a stationary liquid phase (SP 2330, Supelco, Inc., Bellafonte, PA). C17:0 was added as an internal standard to the phospholipids isolated by thin layer chromatography. The detailed methods used for plasma and formula fatty acid analysis have been described previously (37). The molar concentrations of PC and PE were estimated from the molar concentration and percent of total fatty acids as AA in each fraction by assuming ≤1 molecule of AA per molecule of phospholipid.
Statistical analysis. Fisher's test (38) was used to determine whether diet affected the incidence of disease. Repeated-measures ANOVA was used to compare in-hospital weight gain and energy intake before NEC and the effects of diet on the concentrations of plasma phospholipids and their concentrations of AA and DHA.
RESULTS
Incidence of disease. Of the 85 infants in the control group, 15 (17.6%) developed NEC stage II (n = 9) or III (n = 6) (Table 5). Their NEC was diagnosed at a mean of 18.4 d of age (range 12-35 d). Four of the 15 infants with NEC died from the disease. One other control infant without NEC died after surgery for intestinal strictures. Only one of 34 infants (2.9%) fed the experimental formula developed NEC. That infant developed stage III NEC at 18 d of age (Table 5), 12 d after starting the experimental formula, and died.
The incidence of NEC in the experimental group was significantly lower than in the control group (p <0.05) (Table 5). The incidence of NEC in the control group (17.6%) did not appear different from the 22.4% incidence of NEC among very low birth weight infants cared for in our nursery before this study (1). On the other hand, the low incidence of NEC in the experimental group was unprecedented for our nursery. Male infants accounted for a disproportionate number of NEC cases. When male intants fed the control formula were compared with those fed the experimental formula, the effect of diet was highly significant (p < 0.01). Male infants fed the control formula also had a higher incidence of NEC than did female infants fed the control formula (p < 0.05) (Table 5).
Although NEC has been associated with many neonatal and perinatal characteristics, only six independent risk factors were identified in an earlier multicenter study that included our nursery (1): black male, vaginal delivery, mother <25 y, birth weight <1000 g, 5-min Apgar <7, and prolonged ruptured membranes. We reasoned that the infant who developed NEC on the experimental formula might carry a higher number of these risk factors than infants fed the control formula. In fact, the single case of NEC in the experimental group had five of these six risk factors, whereas the control infants with NEC had a range of one to five risk factors and carried a lower mean number of risk factors (Table 6). Antenatal steroids did not appear to reduce the incidence of NEC. Sixty-nine percent of infants with NEC received antenatal steroids compared with 54% of infants who did not develop NEC.
We also looked at PDA and indomethacin exposure because these have been associated with NEC in several studies. Twelve infants developed NEC (11 control, 1 experimental subject) among the 103 (73 control, 30 experimental subjects) infants who did not have PDA and were not exposed to indomethacin (11.7%). Four infants developed NEC (all control subjects) of the 13 infants with PDA (10 control, 3 experimental subjects) (30.8%), two among seven infants who were given indomethacin (5 control, 2 experimental subjects) and two among six infants (5 control, 1 experimental subject) who were not given indomethacin nor surgical ligation. Three other infants (2 control, 1 experimental subject) exposed to indomethacin in utero had neither PDA nor NEC. The type of diet fed in hospital had no influence on the incidence or apparent severity of documented septicemia, retinopathy of prematurity, or BPD (Table 5).
Energy intake and growth. Table 7 shows the energy intake from parenteral and enteral nutrition during hospitalization. There were no differences in total energy intake or progression of enteral feeding between the diet groups that suggested more rapid progression of enteral intake in the group with the higher incidence of NEC (control subjects). Furthermore, diet did not influence weight gain among infants who could be maintained on enteral feeding (Table 8). If anything, enteral intake as a proportion of total energy intake was somewhat higher in the experimental group beginning in the 3rd wk of study (Table 7). Because enteral intakes were advanced as tolerated, the somewhat higher ratio of enteral to parenteral intake that developed in the experimental group well into the study could suggest generally better feeding tolerance before the development of overt disease in some infants.
Concentration of selected lipids. Although AA and DHA were components of the experimental formula, plasma PC AA and DHA concentration changed little between enrollment and 2 wk after full enteral feeding (Fig. 1, A and B). In contrast, the concentrations of plasma PC AA and DHA decreased by approximately 40% in the control group. During the same interval, the total concentration of plasma PC increased by 27.7% in the experimental group but was virtually unchanged in the control group (Fig. 1C).
(A) Plasma PC AA concentrations. (B) Plasma PC DHA concentrations. (C) Plasma total PC concentrations. (D) Plasma PE AA concentrations. (E) Plasma PE DHA concentrations. (F) Plasma total PE concentrations. Control group (•) and experimental group (▴). Study enrollment (E, ∼5 d of age), full enteral feeding (FF), and 2 wk after FF (FF + 2). Data are expressed as the mean ± SD. *Diet groups differ, p <0.05.
The concentration of plasma PE AA increased by 98% between enrollment and 2 wk after full enteral feeding in the experimental group, whereas there was little change in the concentration of PE AA in the control group. (Fig. 1D). DHA declined in both groups but the decrease was larger in the control group (Fig. 1E). The total concentration of PE in the experimental group increased by 40% but there was no effect on PE in the control group (Fig. 1F). The relative effects of diet on the concentrations of PC and PE and on AA and DHA in these phospholipids were similar and highly significant. However, ever, as indicated here and in Figure 1, the specific effects of diet were influenced by the particular phospholipid.
DISCUSSION
The egg phospholipids fed in this study were 75% PC, and they provided nearly equimolar amounts of AA and choline as well as small amounts of DHA. Because AA, choline, and their metabolic products play important roles in gastrointestinal function, it is plausible that one or more of these factors may have protected our study infants against NEC. For example, eicosanoids derived from AA are important regulators of normal gastrointestinal function (39). They act as vasodilators in the mesenteric vascular bed (40), thereby increasing blood flow to the intestine (41). Eicosanoids derived from AA also function as homeostatic regulators of intestinal motility and secretion (39), in cytoprotection of gastrointestinal mucosa (42), and by increasing mucosal growth (43), mucus secretion (44), phospholipid synthesis (45), and the density of surfactant-like particles in the mucus gel layer (46).
Compared with term infants, preterm infants have lower plasma phospholipid AA (47). A relationship between the concentration of AA in plasma phospholipids and somatic growth of preterm infants has been used to suggest that at least some formula-fed preterm infants have suboptimal AA status due to their early birth and diets that do not include AA (48). The suggestion that AA could have been involved in protection against NEC is further supported by an earlier study that found more NEC (though not a statistically significant increase) (49) in preterm infants whose phospholipid AA was decreased by feeding an experimental formula with long chain n-3 fatty acids (50). It is well known that n-3 fatty acids and their lipid-derived mediators have different physiologic functions than does AA and mediators derived from AA. Prostaglandin E2 production depends upon tissue PC AA concentration (51). When the diet contains a balance of AA and DHA, such as the experimental formula fed in this study, there is evidence that tissue eicosanoid profiles favor the n-6 (e.g. AA) rather than n-3 (e.g. DHA) fatty acid family (52). The data of Huang and Craig-Schmidt (52) could be used to suggest that n-6 fatty acid-derived prostaglandins were lower (though not measured) in our previous study (49) relative to the study presented here.
In further support of the hypothesis that prostaglandins from AA may have had a protective role in this study, prostaglandin E1 has been shown to counteract the reduction in mesenteric blood flow and ameliorate the bowel injury in experimental NEC caused by platelet-activating factor (53). Some reports show that preterm infants given indomethacin to permit closure of a PDA have a higher incidence of NEC (54,55). However, the incidence of NEC was not affected when indomethacin was given prophylactically before PDA developed (56) and was lower among indomethacin-treated infants with clinically significant PDA (57). The variability among these reports suggests that a mediating variable, e.g. mesenteric blood flow, may influence the response to indomethacin. More infants with PDA in this study developed NEC than did infants without PDA; however, the proportions of infants on each diet who developed PDA or who were treated for PDA with indomethacin were similar.
The experimental formula provided four times as much choline as the control formula, nearly all as PC. The choline phospholipids (PC and sphingomyelin) are structurally important for all cell membranes, and lysoPC and lysosphingomyelin modulate protein kinase C activity and mediate growth-factor actions, respectively [see Zeisel (58) for review]. Choline is the source of acetylcholine, important for intestinal vasodilation and motility (59), mediating gastric mucus phospholipid secretion (60) and increasing intestinal fluid and ion transport (61). One mechanism by which small arteries in the mesentery dilate is by the binding of acetylcholine to muscarinic M-3 receptors followed by the release of nitric oxide from the arterial endothelium (62). Both nitric oxide (63) and arginine (64), a substrate of nitric oxide synthase, have been shown to protect against intestinal injury in experimental models of NEC caused by, respectively, platelet-activating factor and intraluminal acidified casein.
In choline deficiency, tissue acetylcholine is reduced (65,66). Subsequently, the unmodulated vasoconstrictive effects of adrenergic neurotransmitters may lead to tissue hypoxia and necrosis (65). Instances of heart, liver, kidney, and pancreatic necrosis have been reported in choline-deficient animals (67). Intestinal acetylcholine also decreases in choline deficiency (65), but neither low choline nor low acetylcholine have been associated with intestinal necrosis (67). Even if the intestine were less susceptible than other organs to choline deficiency, choline might be limited for intestinal function under circumstances that increase the need for choline. Choline requirement is influenced by a number of physiologic and nutritional variables including rapid growth/high energy intakes, male gender, intestinal flora, and nutritional status of protein, zinc, folic acid, vitamin B12, and antioxidants (67). Although the control formula had a choline concentration (174 mg or 1436 µmol/L) similar to mature human milk (158 mg or 1351 µmol/L) (68), conditions known to increase the need for choline commonly occur among preterm infants. These include relatively higher energy intakes and growth, marginal protein status and marginal nutritional status in general, and overgrowth of atypical intestinal organisms. Additionally, low vitamin B12 and folic acid status characterize many pregnancies in the lower socioeconomic class (69), especially those ending in preterm birth (70,71), such as the lower socioeconomic class preterm infants studied here.
Another factor that might have influenced the incidence of NEC was that most choline in the experimental formula was PC. Holmes-McNary et al. (68) showed that human milk contains less free choline and more esterified choline than cow's milk-derived infant formulas, although human milk contained mainly phosphocholine and glycerophosphocholine. A study reported later by these authors found that these various sources of choline had different bioavailability in young rats (72).
The experimental formula contained approximately seven times as much choline-containing phospholipids as human milk, mostly PC (68). PC is a major component of pulmonary surfactant, the deficiency of which can lead to respiratory distress syndrome. It is well known that preterm infants have relatively low pulmonary conversion of phosphocholine to CDP-choline, the rate-limiting step in the enzymatic conversion of choline to PC (73). As a result, exogenous surfactants are routinely administered to the airways of preterm infants to treat surfactant deficiency. Recent studies have demonstrated a gastrointestinal surfactant with many similarities to lung surfactant (45,74–77) that protects the gastric mucosa against damage from low pH (77–79). Like the administration of exogenous surfactant into the airways with prevention against respiratory distress syndrome, exogenous phospholipids have been administered enterally and shown to protect against and promote the healing of gastric ulcers (79,80). At present, the function of intestinal surfactant and the ability of preterm infants to synthesize or otherwise maintain intestinal surfactant are unknown. It is interesting to consider that the egg phospholipid-containing formula might have protected the lumenal surface of the intestine in some manner (81), thereby reducing the incidence of NEC.
Antenatal steroids did not appear to protect against NEC as suggested by an earlier study (15), nor were they protective in several other recent reports (1,82,83). Human milk has been shown to lower the incidence of NEC (17), but none of the infants in this study received any human milk by design. The risk factors for NEC identified in an earlier study from the NICHD Neonatal Network (1) were present in the same proportion in each diet group even though NEC was not. Other evidence that the randomization led to a comparison of equivalent groups may be found in Table 4 (similar neonatal/ perinatal characteristics), Table 5 (similar occurrence of disease other than NEC), and Tables 7 and 8 (similar enteral and parenteral nutrition and growth before developing NEC). Because the diet groups appeared to be similar, it is reasonable to conclude that the lower incidence of NEC in the experimental formula was most likely due to the presence of egg phospholipid in that formula.
In summary, we studied diseases in a select group of hospitalized preterm infants to assess the risk/benefit of feeding an infant formula with egg phospholipids. Egg phospholipid-containing formula reduced the incidence of NEC but had no effect on the incidence or severity of other common diseases of hospitalized preterm infants compared with the control formula. The number of infants studied was relatively small, but the effect of diet reached statistical significance because the reduction in NEC with the experimental formula was very large. A type I error cannot be ruled out from a single study; however, there is strong evidence for the importance of several components of egg phospholipids as well as dietary phospholipids themselves in maintenance of intestinal blood flow and cytoprotection of the intestinal mucosa. In preterm infants, these physiologic functions of the gastrointestinal tract and the roles that nutrients play in modulating them are poorly understood (84). However, the association between physiologic immaturity and NEC is well known. We speculate that one or more components of egg phospholipids enhanced one or more immature intestinal functions to lower the incidence of NEC in this study. Based on these data, a large prospective randomized trial to test the effect of this egg phospholipid-containing formula on NEC seems warranted, because NEC and the fear of NEC remain important causes of morbidity and mortality among preterm infants.
Abbreviations
- NEC:
-
necrotizing enterocolitis
- AA:
-
arachidonic acid
- DHA:
-
docosahexaenoic acid
- PC:
-
phosphatidylcholine
- PE:
-
phosphatidylethanolamine
- PDA:
-
patent ductus arteriosus
REFERENCES
Uauy RD, Fanaroff AA, Korones SB, Phillips EA, Phillips JB, Wright LL 1991 Necrotizing enterocolitis in very low birth weight infants: biodemographic and clinical correlates. J Pediatr 119: 630–638
Brown EG, Sweet AY 1982 Neonatal necrotizing enterocolitis. Pediatr Clin North Am 29: 1149–1170
Tobiansky R, Lui K, Roberts S, Beddovi M 1995 Neurodevelopmental outcome in very low birthweight infants with necrotizing enterocolitis requiring surgery. J Paediatr Child Health 31: 233–236
Rand T, Weninger M, Kohlhauser C, Bischof S, Heinc-Peer G, Trattnig S, Popow C, Salzer HR 1996 Effect of umbilical arterial catheterization on mesentehemodynamics. Pediatr Radiol 26: 435–438
Lopez SL, Taeusch HW, Findlay RD, Walther FJ 1995 Time of onset of necrotizing enterocolitis in newborn infants with known prenatal cocaine exposure. Clin Pediatr 34: 424–429
Tesley AM, Merritt TA, Dixon SD 1988 Cocaine exposure in a term neonate. Necrotizing enterocolitis as a complication. Clin Pediatr 27: 547–550
Kuscheid T, Holschneider AM 1993 Necrotizing enterocolitis mortality and long-term results. Eur J Pediatr Surg 3: 139–143
Kliegman RM, Walsh MC 1992 Pathophysiology and epidemiology ofnecrotizing enterocolitis. In: Polin RA, Fox WW (eds) Fetal and Neonatal Physiology, Vol. II. WB Saunders, Philadelphia, 1078–1084.
Ohman U 1984 The effects of luminal distension and obstruction on the intestinal circulation. In: Shepherd AP, Granger DN (eds) Physiology of the Intestinal Circulation. Raven Press, York, 321–334.
Clark DA, Miller MJS 1992 Development of the gastrointestinal circulation in the fetus and newborn. In: Polin RA, Fox WW (eds) Fetal and Neonatal Physiology, Vol. I. WB Saunders, Philadelphia, 690–694.
Beach RC, Menzies IS, Clayden GS, Scopes JW 1982 Gastrointestinal permeability changes in the preterm neonate. Arch Dis Child 57: 141–145
Edelstone D, Holzman I 1984 Fetal and neonatal intestinal circulation. In: Shepherd AP, Granger DN (eds) Physiology of the Intestinal Circulation. Raven Press, New York, 179–190.
Hoy C, Millar MR, MacKay P, Godwin PGR, Langdale V, Levene MI 1990 Quantitative changes in faecal microflora preceding necrotising enterocolitis in premature neonates. Arch Dis Child 65: 1057–1059
Caplan MS, MacKendrick W 1993 Necrotizing enterocolitis: a review of pathogenetic mechanisms and implications for prevention. Pediatr Pathol 13: 357–369
Bauer CR, Morrison JC, Poole WK, Korones SB, Boehm JJ, Rigatto H, Zachman RD 1984 A decreased incidence of necrotizing enterocolitis after prenatal glucocorticoid therapy. Pediatrics 73: 682–688
Halac E, Halac J, Begue EF, Casanas JM, Indiveri DR, Petit JF, Figueroa MJ, Olmas JM, Rodriquez LA, Obregon RJ, Martinez MV, Grinblat DA, Vilarrodona HO 1990 Prenatal and postnatal corticosteroid therapy to prevent neonatal necrotizing enterocolitis: a controlled trial. J Pediatr 117: 132–138
Lucas A, Cole TJ 1990 Breast milk and neonatal necrotising enterocolitis. Lancet 336: 1519–1523
Carrion V, Egan EA 1990 Prevention of neonatal necrotizing enterocolitis. J Pediatr Gastroenterol Nutr 11: 317–323
Eibl MM, Wolf HM, Furnkranz H, Rosenkranz A 1988 Prevention of necrotizing enterocolitis in low-birth-weight infants by IgA-IgG feeding. N Engl J Med 319: 1–7
Grylack LJ, Scanlon JW 1978 Oral gentamicin therapy in the prevention of neonatal necrotizing enterocolitis. Am J Dis Child 132: 1192–1194
Egan EA, Mantilla G, Nelson RM, Eitzman DV 1976 A prospective controlled trial of oral kanamycin in the prevention of neonatal necrotizing enterocolitis. J Pediatr 89: 467–470
Israel EJ, Schiffrin EJ, Carter EA, Frieberg E, Walker WA 1991 Cortisone strengthens the intestinal mucosal barrier in a rodent necrotizing enterocolitis model. Adv Exp Med Biol 310: 375–380
Goldman AS, Smith CW 1979 Host resistance factors in human milk. J Pediatr 94: 295–296
Carver JD, Cox WI, Barness LA 1990 Dietary nucleotide effects upon murine natural killer cell activity and macrophage activation. J Parenter Enteral Nutr 14: 18–22
Insoft RM, Sanderson IR, Walker WA 1996 Development of immune function in the intestine and its role in neonatal diseases. Pediatr Clin North Am 43: 551–571
Carver JD, Barness LA 1996 Trophic factors for the gastrointestinal tract. Clin Perinatol 23: 265–285
Egan EA, Nelson RM, Mantilla G, Eitzman DV 1977 Additional experience with routine use of oral kanamycin prophylaxis for necrotizing enterocolitis in infants under 1000 g. J Pediatr 93: 31–32
Boyle R, Nelson JS, Stonestreet BS, Peter G, Oh W 1978 Alterations in stool flora resulting from oral kanamycin prophylaxis of necrotizing enterocolitis. J Pediatr 93: 857–861
Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R 1991 New Ballard Score expanded to include extremely premature infants. J Pediatr 119: 417–423
Lubchenco LO, Hansman C, Boyd E 1966 Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 wk. Pediatrics 37: 403–408
Putnam JC, Carlson SE, DeVoe PW, Barness LA 1982 The effect of variations in dietary fatty acids on the fatty acid composition of erythrocyte phosphatidylcholine and phosphatidylethanolamine in human infants. Am J Clin Nutr 36: 106–114
Walsh MC, Kliegman RM 1986 Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 33: 179–201
Bell MJ, Ternberg JL, Feigin RD, Keating JP 1978 Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg 187: 1–7
Pourcyrous M, Bada HS, Korones SB, Baselski V, Wong SP 1993 Significance of serial C-reactive protein responses in neonatal infection and other disorders. Pediatrics 92: 431–435
Northway WH Jr, Rosan RC, Porter DY 1967 Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 276: 357–374
Committee for the Classification of ROP 1984 An International classification of retinopathy of prematurity. Pediatrics 74: 27–33
Carlson SE, Ford AJ, Werkman SH, Peeples JM, Koo WWK 1996 Visual acuity and fatty acid status of term infants fed human milk and formula with and without docosahexaenoate and arachidonate from egg yolk lecithin. Pediatr Res 39: 1–7
Matthews DE, Farewell VT 1988 Using and Understanding Medical Statistics, 2nd Ed. S Karger AG, Basel, Switzerland, 20–38.
Whittle BJR, Vane JR 1987 Prostanoids as regulators of gastrointestinal function. In: Johnson LR, (ed) Physiology of the Gastrointestinal Tract, 2nd Ed. Raven Press, New York, 143–180.
Chapnick BM, Feigen LP, Hyman AL, Kadowitz PJ 1978 Differential effects of prostaglandins in the mesenteric vascular bed. Am J Physiol 235: H326–H332
Einzig S, Rao GHR, White JG 1980 Differential sensitivity of regional vascular beds in the dog to low-dose prostacyclin infusion. Can J Physiol Pharmacol 58: 940–946
Wilson DE 1991 Role of prostaglandins in gastroduodenal mucosal protection. J Clin Gastroenterol 13: S65–S71
Hart MH, Grandjean CJ, Park JHY, Erdman SH, Vanderhoof JA 1988 Essential fatty acid deficiency and postresection mucosal adaptation in the rat. Gastroenterology 94: 682–687
Allen A, Flemstrom G, Garner A, Kivilasskso E 1993 Gastroduodenal mucosal protection. Physiol Rev 73: 823–857
Scheiman JM, Kraus ER, Bonnville LA, Weinhold PA, Boland CR 1991 Synthesis and prostaglandin E2-induced secretion of surfactant phospholipid by isolated gastric mucous cells. Gastroenterology 100: 1232–1240
Kao Y-CJ, Lichtenberger LM 1990 A method to preserve extracellular surfactant-like phospholipids on the luminal surface of the rodent gastric mucosa. J Histochem Cytochem 38: 427–431
Carlson SE 1996 Arachidonic acid status of human infants: Influence of gestational age at birth and diets with very long chain n-3 and n-6 fatty acids. J Nutr 16: 1092S–1098S
Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA 1993 Arachidonic acid status correlates with first year growth of preterm infants. Proc Natl Acad Sci USA 90: 1073–1077
Carlson SE, Werkman SH, Tolley EA 1996 The effect of long chain n-3 fatty acid supplementation on visual acuity and growth of preterm infants with and without bronchopulmonary dysplasia. Am J Clin Nutr 63: 687–697
Carlson SE, Cooke RJ, Rhodes PG, Peeples JM, Werkman SH, Tolley EA 1991 Long-term feeding of formulas high in linolenic acid and marine oil to very low birth weight infants: phospholipid fatty acids. Pediatr Res 30: 404–412
Kawashima Y, Mizuguchi H, Kozuka H 1994 Modulation by dietary oils and clofibric acid of arachidonic acid content in phosphatidylcholine in liver and kidney of rat: effects on prostaglandin formation in kidney. Biochim Biophys Acta 1210: 187–194
Huang M-C, Craig-Schmidt MC 1996 Arachidonate and docosahexaenoate added to infant formula influenced fatty acid composition and subsequent eicosanoid production in neonatal pigs. J Nutr 126: 2199–2208
Zhang C, Hsueh W 1991 PAF-induced bowel necrosis: effects of vasodilators. Dig Dis Sci 36: 634–640
Norton ME, Merrill J, Cooper BA, Kuller JA, Clyman RI 1993 Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med 329: 1602–1607
Major CA, Lewis DF, Harding JA, Porto MA, Garite TJ 1994 Tocolysis with indomethacin increases the incidence of necrotizing enterocolitis in the low-birth-weight neonate. Am J Obstet Gynecol 170: 102–106
Couser RJ, Ferrara TB, Wright GB, Cabalka AK, Schilling CG, Hoekstra RE, Payne NR 1996 Prophylactic indomethacin therapy in the first twenty-four hours of life for the prevention of patent ductus arteriosus in preterm infants treated prophylactically with surfactant in the delivery room. J Pediatr 128: 631–637
Robie DK, Waltrip T, Garcia-Prats JA, Pokorny WJ, Jaksic T 1996 Is surgical ligation of a patent ductus arteriosus the preferred initial approach for the neonate with extremely low birth weight? J Pediatr Surg 31: 1134–1137
Zeisel SH 1994 Choline and human nutrition. Annu Rev Nutr 14: 269–296
Bean JW, Sidky MM 1958 Intestinal blood flow as influenced by vascular and motor reactions to acetylcholine and carbon dioxide. Am J Physiol 194: 512–518
Sengupta S, Piotrowski E, Slomiany A, Slomiany BL 1991 Role of adrenergic and cholinergic mediators in gastric mucus phospholipid secretion. Biochem Int 24: 1145–1153
Cooke HJ 1987 Neutral and humoral regulation of small intestine electrolyte transport. In: Johnson LR (ed) Physiology of the Gastrointestinal Tract, 2nd Ed. Raven Press, New York, 1307–1350.
Wu CC, Chen SJ, Yen MH 1997 Loss of acetylcholine-induced relaxation by M-3 receptor activation in mesenteric arteries of spontaneously hypertensive rats. J Cardiovasc Pharmacol 30: 245–252
MacKendrick W, Caplan M, Hsueh W 1993 Endogenous nitric oxide protects against platelet-activating factor-induced bowel injury in the rat. Pediatr Res 34: 222–228
Di Lorenzo M, Bass J, Krantis A 1995 Use of L-arginine in the treatment of experimental necrotizing enterocolitis. J Pediatr Surg 30: 235–241
Nagler AL, Dettbarn W-D, Seifter E, Levenson SM 1968 Tissue levels of acetyl choline and acetyl cholinesterase in weanling rats subjected to acute choline deficiency. J Nutr 94: 13–19
Nagler AL, Dettbarn W-D, Levenson SM 1968 Tissue levels of acetylcholine and acetylcholinesterase in weanling and germfree rats subjected to acute choline deficiency. J Nutr 95: 603–606
Wilson RB 1978 Nutrient deficiencies in animals: Choline. In: Rechcicl M Jr (ed) Handbook Series in Nutrition and Food, Section E: Nutritional Disorders, Vol. II. CRC Press, West Palm Beach, FL, 95–121.
Holmes-McNary MQ, Cheng W-L, Mar M-H, Fussell S, Zeisel SH 1996 Choline and choline esters in human milk and rat milk and in infant formulas. Am J Clin Nutr 64: 572–576
Bailey LM, Mahan CS, Dimperio D 1997 Folacin and iron status in low-income pregnant adolescents and mature women. Am J Clin Nutr 33: 1997–2001
Carlson SE, Palmer SM, Caldwell EI, Rhodes PG 1985 Folate status and length of gestation. Am J Clin Nutr 41: 844
O'Scholl T, Hediger ML, Schal JI, Khoo C-S, Fischer RL 1996 Dietary and serum folate: their influence. Am J Clin Nutr 63: 520–525
Cheng W-L, Holmes-McNary MQ, Mar M-H, Lien EL, Zeisel SH 1996 Bioavailability of choline and choline esters from milk in rat pups. J Nutr Biochem 7: 457–464
Farrell PM, Epstein MF, Fleischman AR, Oakes GK, Chez RA 1976 Lung lecithin biosynthesis in the nonhuman primate fetus: determination of the primary pathway in vivo. Biol Neonate 29: 238–246
Wassef MK, Lin YN, Horowitz MJ 1979 Molecular species of phosphatidylcholine from rat gastric mucosa. Biochim Biophys Acta 573: 222–226
Butler BD, Lichtenberger LM, Hills BA 1983 Distribution of surfactants in the canine GI tract and their ability to lubricate. Am J Physiol 7: G645–G651
Mack DR, Neumann AW, Policova Z, Sherman PM 1992 Surface hydrophobicity of the intestinal tract. Am J Physiol 262: G171–G177
Lichtenberger LM 1995 The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol 57: 565–583
Goddard PJ, Lichtenberger LM 1987 Does aspirin damage the canine gastric mucosa by reducing its surface hydrophobicity? Am J Physiol 15: G421–G430
Lichtenberger LM, Graziani LA, Dial EJ, Butler BD, Hills BA 1983 Role of surface-active phospholipids in gastric cytoprotection. Science 219: 1327–1329
Swarm RA, Ashley SW, Soybel DI, Ordway FS, Cheung LY 1987 Protective effect of exogenous phospholipid on aspirin-induced gastric mucosal injury. Am J Surg 153: 48–53
Bengmark, S., Jeppsson, B 1995 Gastrointestinal surface protection and mucosa reconditioning. J Parenter Enterol Nutr 19: 410–415
Moise AA, Weardon ME, Kozinetz CA, Gest AL, Welty SE, Hansen TN 1995 Antenatal steroids are associated with less need for blood pressure support in extremely premature infants. Pediatrics 95: 845–850
Tucker L, Hoff C, Peevy K, Brost B, Holland S, Calhoun BC 1995 The effects of antenatal steroid use in premature rupture of membranes. Aust NZ J Obstet Gynaecol 35: 390–392
Kleigman RM, Walker WA, Yolken RH 1993 Necrotizing enterocolitis: research agenda for a disease of unknown etiology and pathogenesis. Pediatr Res 34: 701–708
Engelhardt, R, Winzeler, J 1993 Automated and rapid determination of choline in nutritional products employing immobilized choline oxidase and electrochemical detection of hydrogen peroxide. Institute of Food Technologists Annual Meeting Technical Program Book of Abstracts, 120
Engelhardt, R, Vojacek, M 1996 Determination of total choline in nutritional products. 9th Annual Meeting of the Central Section Association of Official Analytical Chemist, 9
Acknowledgements
The authors thank Jeanette Peeples of the University of Tennessee, Memphis, for the plasma phospholipid fatty acid analyses and Rene Engelhardt of Ross Products Division for choline analyses.
Author information
Authors and Affiliations
Additional information
Supported by the National Institute of Child Health and Human Development (R01-HD31329) and a gift from Ross Products Division, Abbott Laboratories (Columbus, OH).
Rights and permissions
About this article
Cite this article
Carlson, S., Montalto, M., Ponder, D. et al. Lower Incidence of Necrotizing Enterocolitis in Infants Fed a Preterm Formula with Egg Phospholipids. Pediatr Res 44, 491–498 (1998). https://doi.org/10.1203/00006450-199810000-00005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199810000-00005
This article is cited by
-
Fish oil- and soy oil-based lipid emulsions in neonatal parenteral nutrition: a systematic review and meta-analysis
European Journal of Clinical Nutrition (2016)
-
Daily Enteral DHA Supplementation Alleviates Deficiency in Premature Infants
Lipids (2016)
-
Beyond building better brains: bridging the docosahexaenoic acid (DHA) gap of prematurity
Journal of Perinatology (2015)
-
Long-chain polyunsaturated fatty acids attenuate the IL-1β-induced proinflammatory response in human fetal intestinal epithelial cells
Pediatric Research (2015)
-
Human Milk is the Feeding Strategy to Prevent Necrotizing Enterocolitis
Current Pediatrics Reports (2014)