Abstract
Zellweger syndrome is a prototype of peroxisomal biogenesis disorders and a fatal autosomal recessive disease with no effective therapy. We identified nine genetic complementation groups of these disorders, and mutations in peroxisome assembly factor-1 (PAF-1) and the 70-kD peroxisomal membrane protein (PMP70) genes have been detected by our group F and Roscher's group 1, respectively. We now describe permanent recovery from generalized peroxisomal abnormalities in fibroblasts of a Zellweger patient from group F, such as biochemical defects of peroxisomal β-oxidation, plasmalogen biosynthesis, and morphologic absence of peroxisomes, by stable transfection of human cDNA encoding PAF-1. In the light of these observations, we designed a gene expression system using fibroblasts from patients with peroxisomal biogenesis disorders. In Zellweger fibroblasts obtained from Roscher's group 1 and transfected with human cDNA encoding PMP70, peroxisomes were not morphologically identifiable, and peroxisomal function did not normalize.
Similar content being viewed by others
Main
Patients with ZS (McKusick 214100)(1) have profound hypotonia from birth, seizures, absence of deep tendon reflexes, dysmorphic features, and skeletal abnormalities. Demyelination and neuronal heterotopia are prominent, along with hepatic fibrosis, renal cortical cysts, and failure to thrive. This disorder is characterized by the absence of peroxisomes and is regarded as a prototype of PBD, such as NALD and IRD. Although a number of metabolic defects, including the β-oxidation system, plasmalogen biosynthesis, and oxidation of phytanic acid and pipecolic acid seem to be linked to this syndrome, the primary etiology has only recently been described. Genetic heterogeneity in PBD was investigated in various laboratories by complementation analysis. We identified eight complementation groups in these disorders (group A, B, C = 4, D, E = 1, 2, 3, and 6), by means of cell fusion and immunofluorescence analysis, in collaboration with the Kennedy Institute(2). Tsukamoto et al.(3) cloned, by functional complementing assay, a rat cDNA encoding PAF-1 that restores the assembly of peroxisomes in Z65, one of the peroxisome-deficient CHO cell mutants(4). Later a female infant MM was diagnosed as a case of typical ZS and was classified into a ninth new complementation group of PBD (group F). As a result of cell-fusion of her fibroblasts and Z65, these two cell lines probed to be of the same complementation group. We found that rat and human PAF-1 cDNA restored the assembly of peroxisomes in her fibroblasts when mammalian expression vector pcD2 was used, and that there was a nonsense mutation of the PAF-1 gene she carried(5).
Afterward, Gärtner et al.(6) reported that two ZS patients belonged to Roscher's complementation group 1, and that a different mutation was seen in only one allele of human PMP70 cDNA, respectively. They suggested that PMP70 was the gene responsible for PBD(6). In the present work, we asked whether human PAF-1 and PMP70 would restore biochemical abnormalities in ZS fibroblasts of complementation group F and 1, respectively. We used a calcium phosphate transfection protocol and a neo marker expression vector(7).
METHODS
Cell lines. Fibroblasts MM and RF were obtained from Japanese female infants with ZS belonging to group F in PBD. The disorder in MM was caused by a nonsense mutation in the PAF-1 gene(5). ZS9(GM06094), ZS10(GM00228), ZS11(GM06256), ZS12(GM08040), IRD2 (GM08770), IRD3(GM08772), and IRD4(GM08769) were purchased from The Human Genetic Mutant Cell Repository (Camden, NJ), and all of these belonged to Roscher's group 1 or our group E in PBD complementation grouping(2). SV-MM and SV-ZS12 were transformed by transfecting SV40 ori- DNA with the use of an electroporator into the fibroblasts MM and ZS12, respectively(8).
Transfection of cells. Fibroblasts cells (5 × 105) were cultured in 100-mm dishes and transfected with human PAF-1(5) or PMP70 cDNA(9), using a mammalian expression vector pcD2(5). Stable transfectants were obtained by transfection into SV40-transformed human fibroblasts and selection for 8 wk in growth medium containing 400 μg/mL of G418.
Immunofluorescence staining. The cells were selected in G418 medium for 7 d after transfection, cultivated on coverslips, and fixed with 4% paraformaldehyde/0.1 M potassium phosphate buffer, pH 7.4. Peroxisomes were immunocytochemically stained with rabbit anti-human catalase IgG, as previously described(10).
Biochemical assessments of peroxisomal enzyme. Immunoblot analysis of peroxisomal 3-ketoacyl-CoA thiolase(11), and measurements of lignoceric acid oxidation(12) and DHAP-AT activities(13) were done, as previously described.
RESULTS
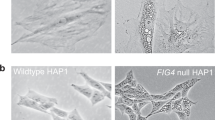
Immunofluorescence staining. After transfection with human PAF-1 cDNA into fibroblasts MM and RF, 60% of the cells (12 of 20 G418-resistant cells on the average) contained numerous peroxisomes, as seen in duplicate experiments, respectively. On the other hand, in all seven PBD cell lines belonging to Roscher's group 1 (ZS 9-12 and IRD 2-4) biogenesis of peroxisomes was not restored immunocytochemically, by transfection with the human PMP70 cDNA (Table 1). Furthermore, in the stable transfectants obtained by transfection with human PAF-1 cDNA into SV-MM, biogenesis of peroxisomes was also restored immunocytochemically (Fig. 1A). On the other hand, there was no peroxisome in the stable transfectants obtained by transfection with human 70PMP cDNA into SV-ZS12 (Fig. 1B).
Lignoceric acid oxidation and DHAP-AT activities. Lignoceric acid oxidation activity in the stable transfectants with human PAF-1 cDNA into SV-MM restored about 60% of activity in the control (238 ± 98 pmol/mg of protein/h), whereas in the SV-MM before transfection, the activity was 10% of that in the control (Fig. 2). The DHAP-AT activity in the stable transfectants with human PAF-1 cDNA into the SV-MM also restored half the activity in the control (4.63 ± 0.35 nmol/mg of protein/h), whereas in the SV-MM, the activity was below 5% of that in the control (Fig. 3). On the other hand, neither lignoceric acid oxidation nor DHAP-AT activity in the stable transfectants with human 70PMP cDNA into the SV-ZS12 was increased, in comparison with findings in untransfected SV-ZS12 (Figs. 2 and 3).
Lignoceric acid oxidation activity of stable transfectants with human PAF-1 and PMP70 cDNA into fibroblasts from our complementation group F (SV-MM) and Roscher's group 1 (SV-ZS12), respectively. SV-MM PAF-1 and SV-ZS12 PMP70 were stable transfectants with the PAF-1 and PMP70 cDNA into the SV-MM and SV-ZS12, respectively.
Immunoblot analysis (Fig. 4). 3-Ketoacyl-CoA thiolase seen in control fibroblasts was of mature size (41 kD) of the subunit (lane 2, arrowhead), yet only precursor size (44 kD) of the subunit which was slightly higher in molecular weight than the mature enzyme was seen in cells SV-MM (lane 3) and SV-ZS12 (lane 5). In transfectants with the PAF-1 cDNA into SV-MM, both mature size(lane 4, arrowhead) and precursor size of the subunit were evident, although only precursor size of the subunit was seen in transfectants with PMP70 cDNA into the SV-ZS12 (lane 6).
Immunoblot analysis of peroxisomal 3-ketoacyl-CoA thiolase in fibroblasts. Lane 1, Purified rat 3-ketoacyl-CoA thiolase (2 ng). Extracts of fibroblasts containing 30 μg of protein, from control (lane 2), SV-MM (lane 3), stable transfectants with the PAF-1 into the SV-MM (lane 4), SV-ZS12 (lane 5), and the transfectants with the PMP70 into the SV-ZS12 (lane 6) were applied.
DISCUSSION
Patients with peroxisomal disorders, including intractable neurogenic diseases such as adrenoleukodystrophy and the more benign forms of PBD are candidates for gene therapy. In previous studies, we cloned human PAF-1 cDNA which restored the assembly of peroxisomes in fibroblasts from a ZS patient of complementation group F in PBD. In the present study, we obtained stable transfectants with the human PAF-1 cDNA into the patient's fibroblasts, using a pcD2 expression vector, and analyzed peroxisomal function of these transfectants. Peroxisomal dysfunctions such as lignoceric acid oxidation, DHAP-AT activity, and processing of peroxisomal 3-ketoacyl-CoA thiolase were restored in the transfectants, as was morphology of the peroxisomes. Therefore, this cultured cellular system was effective for attaining permanent correction of PBD fibroblasts. With use of the pcD2 vector, a 50-60% biochemical restoration occurred. Further experiments using different expression vectors are necessary, and this system will serve for further study on development the effective expression vectors for gene therapy of peroxisomal disorders.
Gärtner et al.(6) found two mutant PMP70 alleles in single ZS probands from the Roscher's complementation group 1, and they suggested that PMP70 was the gene responsible for PBD. In the present study, we found that human PMP70 cDNA did not restore the assembly of peroxisomes in fibroblasts from seven different PBD patients of Roscher's group 1. Furthermore, in a stable transfectant with PMP70 into SV-ZS12 belonging to group 1, there was no evidence of peroxisome assembly. We have not done experiments for biochemical correction in two cell lines where mutations of PMP70 were found by Gärtner et al., therefore, further investigations are needed on the gene responsible for PBD of group 1. We are now cloning this gene, using the CHO mutant, which is of the same complementation group as the human PBD group 1(14). The gene responsible for another complementation group of PBD (Roscher's group 2) was cloned by Dodt et al.(15).
Abbreviations
- ZS:
-
Zellweger syndrome
- PBD:
-
peroxisomal biogenesis disorders
- NALD:
-
neonatal adrenoleukodystrophy
- IRD:
-
infantile Refsum disease
- PAF-1:
-
peroxisome assembly factor-1
- CHO:
-
Chinese hamster ovary
- PMP70:
-
70-kD peroxisomal membrane protein
- DHAP-AT:
-
dihydroxyacetone phosphate acyltransferase
References
Bowen P, Lee CSN, Zellweger H, Lindenberg R 1964 A familial syndrome of multiple congenital defects. Bull Johns Hopkins Hosp 114: 402–414.
Yajima S, Suzuki Y, Shimozawa N, Yamaguchi S, Orii T, Fujiki Y, Osumi T, Hashimoto T, Moser HW 1992 Complementation study of peroxisome-deficient disorders by immunofluorescence staining and characterization of fused cells. Hum Genet 88: 491–499.
Tsukamoto T, Miura S, Fujiki Y 1991 Restoration by a 35K membrane protein of peroxisome assembly in a peroxisome deficient mammalian cell mutant. Nature 350: 77–81.
Tsukamoto T, Yokota S, Fujiki Y 1990 Isolation and characterization of Chinese hamster ovary cell mutants defective in assembly of peroxisomes. J Cell Biol 110: 651–660.
Shimozawa N, Tsukamoto T, Suzuki Y, Orii T, Shirayoshi Y, Mori T, Fujiki Y 1992 A human gene responsible for Zellweger syndrome that affects peroxisome assembly. Science 255: 1132–1134.
Gartner J, Moser HW, Valle D 1992 Mutations in the 70K peroxisomal membrane protein gene in Zellweger syndrome. Nature Genet 1: 16–23.
Chen C, Okayama H 1987 High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7: 2745–2752.
Okamoto H, Suzuki Y, Shimozawa N, Yajima S, Masuno M, Orii T 1992 Transformation and characterization of mutant human fibroblasts defective in peroxisome assembly. Exp Cell Res 201: 307–312.
Kamijo K, Kamijo T, Ueno I, Osumi T, Hashimoto T 1992 Nucleotide sequence of the human 70 kDa peroxisomal membrane protein: a member of ATP-binding cassette transporters. Biochim Biophys Acta 1129: 323–327.
Suzuki Y, Yamaguchi S, Orii T, Tsuneoka M, Tashiro Y 1990 Nonspecific lipid transfer protein (sterol carrier protein 2) defective in patients with deficient peroxisomes. Cell Struct Funct 15: 301–308.
Shimozawa N, Suzuki Y, Orii T, Hashimoto T 1988 Immunoblot detection of enzyme proteins of peroxisomal -oxidation in fibroblasts, amniocytes, and chorionic villous cells: possible marker for prenatal diagnosis of Zellweger syndrome. Prenatal Diagn 8: 287–290.
Suzuki Y, Shimozawa N, Yajima S, Yamaguchi S, Orii T, Hashimoto T 1991 Effects of sodium 2-[5-(4-chlorophenyl) pentyl]-oxirane-2-carboxylate (POCA) on fatty acid oxidation in fibroblasts from patients with peroxisomal diseases. Biochem Pharmacol 41: 453–456.
Shimozawa N, Suzuki Y, Orii T, Yokota S, Hashimoto T 1988 Biochemical and morphologic aspects of peroxisomes in the human rectal mucosa: diagnosis of Zellweger syndrome simplified by rectal biopsy. Pediatr Res 24: 723–727.
Shimozawa N, Tsukamoto T, Suzuki Y, Orii T, Fujiki Y 1992 Animal cell mutants represent two complementation groups of peroxisome-defective Zellweger syndrome. J Clin Invest 90: 1864–1870.
Dodt G, Braverman N, Wong C, Moser A, Moser HW, Watkins P, Valle D, Gould SJ 1995 Mutations in the PTS1 receptor gene, PXR1, define complementation group 2 of the peroxisome biogenesis disorders. Nat Genet 9: 115–125.
Acknowledgements
The authors thank M. Ohara for critical comment.
Author information
Authors and Affiliations
Additional information
Supported in part by a Grant-in-Aid for Scientific Research (06670782) from the Ministry of Education, Science and Culture of Japan, by a Research Grant from the National Center of Neurology and Psychiatry of the Ministry of Health and Welfare of Japan, and by a Research Grant from Uehara Memorial Life Science Foundation and National Children's Hospital.
Rights and permissions
About this article
Cite this article
Shimozawa, N., Suzuki, Y., Tomatsu, S. et al. Correction by Gene Expression of Biochemical Abnormalities in Fibroblasts from Zellweger Patients. Pediatr Res 39, 812–815 (1996). https://doi.org/10.1203/00006450-199605000-00011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199605000-00011