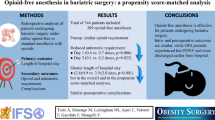
Abstract
Background
Opioids have historically been a first-line therapy for surgical pain control. They were considered optimum and the mainstay of balanced anesthesia, but recently, concerns about their side effects have been raised. The concept of opioid free anesthesia (OFA) was introduced to provide a safer alternative that would provide benefits as well as enhance recovery after surgery.
Results
Sixty patients were enrolled in the study, 30 patients in each group. The two groups, TBA and OFA, were comparable in demographic data (age, sex, body mass index (BMI), lean body weight (LBW)) and duration of surgery. The TBA group showed a statistically significant reduction in the time needed for extubation (P value 0.018) and reaching an Aldrete score of 9 (P value 0.02). There was a significant decrease in pain scores, and nalbuphine consumption in the OFA group that extended to 24 h post-operative.
Conclusions
OFA has a better profile than TBA with regard to post-operative pain score and opioid consumption post-operative, but they have a relative increase in time to extubation and time to reach an Aldrete score of 9.
Similar content being viewed by others
Background
The need for effective pain control is rising nowadays as pain is viewed as an important vital sign and is even considered the fifth vital sign and has been equated with quality control in the health system (Thota et al., 2019).
Although pain is a subjective experience and needs a conscious patient to report, this does not mean that pain does not occur under anesthesia. Increased heart rate and elevated blood pressure in response to surgical stimuli, together with elevated “fight or flight” response biomarkers such as epinephrine and cortisol, are clinical evidence that a patient is suffering. Controlling the autonomic nervous system response to surgical stimuli is one of the main requirements of anesthesia and is often referred to as analgesia. Also, opioids are usually the drugs of choice to achieve hemodynamic stability intra-operatively, and they belong to the group of analgesics (Egan, 2019).
Opioids are still an important component of anesthesia and offer effective analgesia at a low cost, but this is not always the case. Opioids have a better effect on resting pain than pain associated with activity, and their use in high doses could lead to tolerance or hyperalgesia. Also, opioids share various side effects that could affect patient satisfaction and length of stay and make their role in functional recovery questionable in patients with persistent post-operative pain (Echeverria-Villalobos et al., 2020).
Side effects of opioids could lead to significant morbidity and even mortality due to their effect on conscious level, respiratory drive, nausea, vomiting, and constipation. Additionally, there is evidence that opioids could affect the outcome of surgery by increasing the risk of infection or increasing the risk of metastasis in patients suffering from cancer owing to their immunosuppressive effect (Samuels et al., 2017).
Opioid-induced respiratory depression (OIRD) is the most serious side effect of opioids and is also the main cause of opioid-related mortality. It was shown that the risk of respiratory depression is increased in patients suffering from cardiac disease, pulmonary disease, or Obstructive Sleep Apnea (OSA) and with the administration of higher doses of opioids (Gupta et al., 2018).
All of these effects interfere with the post-operative recovery, contribute to a negative perioperative experience, and add to the cost of health care (Gupta et al., 2020).
These effects are more obvious in the obese population owing to the fact that they show a higher incidence of cardiac and pulmonary diseases as well as OSA prevalence (Ortiz and Kwo 2015).
Patient harm from opioids may continue for a long time, as in the case of opioid misuse disorder. The perioperative period was the first episode of opioid consumption for many of these patients. Whenever possible, perioperative opioids should be minimised or eliminated to reduce harm. OFA has gained popularity nowadays as a tool offering equivalent intra-operative hemodynamic stability compared to that of an opioid, with better pain control post-operatively, a lower incidence of nausea and vomiting, and better patient satisfaction. OFA is a technique that provides anesthesia without opioids, either systemic, neuroaxial, or tissue infiltration (Soffin et al., 2019).
An effective OFA strategy consists of a combination of pharmacological agents and non-pharmacological techniques that target different pathways of the pain mechanism (Echeverria-Villalobos et al., 2020).
The key concept in designing a multimodal strategy for pain control is targeting multiple different neurotransmitters and neural relays in the ascending and descending pathways of the nociceptive system simultaneously by anti-nociceptive agents that can act to disrupt information processing (Brown et al., 2018).
The concept of enhanced recovery after surgery (ERAS) was first introduced by the colorectal surgeon, Kehlet, in 1997. He published a paper that suggested the use of evidence-based interventions targeted at the perioperative period to improve patient outcomes (Greenshields and Mythen, 2020).
ERAS protocols intend to reduce the physiological response to surgical stimuli, and they make use of new evidence that comes to light to reduce complications. There is a sufficient body of evidence that links ERAS protocol implementation to a reduction in opioid consumption (Echeverria-Villalobos et al., 2020).
The current study was done to measure the efficacy of opioid free general anesthesia in achieving Enhanced Recovery After Surgery (ERAS) in laparoscopic bariatric surgery and to provide a safer alternative that would provide benefits to patients.
Methods
After receiving ethical approval (FMASU M D 182/2019) from the Research Ethical Committee, in our institute this interventional, randomized, and double–blind controlled trial was conducted in the institute hospitals.
-
Study population
Inclusion criteria: Patients undergoing scheduled laparoscopic bariatric surgery under general anesthesia with a BMI between 40 and 59.9 kg/m2 and an age between 18 and 65 years old.
Exclusion criteria: ASA >3, or whenever there is a contraindication or allergy to dexmedetomidine, ketamine, or fentanyl.
-
Sample size: Using the STATA program, setting alpha error at 5% and power at 80%, the results from previous study (El Sayed et al., 2017) showed that the mean extubation time in the opioid group was 8.92±2.02 while among the opioid free it was 7.65±1.11. Based on this, the needed sample size was 25 cases per group. Each group included 30 patients, taking into account possible dropouts 20%.
In the anesthesia clinic, an informed written consent was taken from every patient 1 day before the surgery. The VAS score was explained to the patients.
Anesthesia was provided according to the hospital protocol with respect to preoperative investigations, fasting hours, and intra-operative monitoring and drugs.
Patients were randomly allocated by computer generated randomization and using opaque sealed envelopes to one of the two groups according to the used analgesia:
Group TBA: Traditional balanced anesthesia
Analgesia was offered by fentanyl as a bolus then by intravenous infusion during maintenance according to Ideal Body Weight (IBW).
Group OFA: Opioid free anesthesia
Analgesia was offered by syringe containing ketamine and dexmedetomidine as a bolus then by intravenous infusion during maintenance according to IBW for ketamine and Total Body Weight (TBW) for dexmedetomidine.
-
IBW was calculated according to the gender-specific Acute Respiratory Distress Syndrome Network (ARDSnet) formulas to calculate IBW (Acute Respiratory Distress Syndrome Network et al., 2000).
-
In men, IBW was calculated as 50 + (0.91 [height in [height in centimeters] x 152.4]).
-
In women, 5 + (0.91 [height in centimeters])
-
After a proper assessment of the airway and anticipation of a difficult airway, all patients received midazolam 2 mg IV for sedation, and pre-oxygenation began with 100% O2 on 8 L/min via face mask for 3 min.
-
All patients received general anesthesia induction by propofol 1.5–2 mg/kg lean body weight (LBW), analgesia according to patient group, lidocaine 1.5 mg/kg (IBW), and muscle relaxation by rocuronium 0.5 mg/Kg IBW. An endotracheal tube was inserted. Anesthesia was maintained by isoflurane whose concentration was adjusted according to the patient’s hemodynamics.
-
LBW was calculated according to the Janmahasatian equation (Janmahasatian et al., 2005)
-
In men, it is calculated as (9270 * Total Body Weight [kg])/(6680 + (216 * BMI [kg/m2])
-
In women, it is calculated as (9270 * Total Body Weight [kg])/(8780 + (244 * BMI [kg/m2]).
-
The analgesia used in the study was prepared in the pharmacy and was given a code so that the anesthetist in charge was blind to the analgesia used. The analgesia was prepared as follows:
-
Fentanyl 2 μg/kg (IBW) as a bolus dose in a 10 ml syringe to be given over 10 min, and the same dose was added to a 50-ml syringe adjusted to a rate of 25 ml/h (1 μg/kg/h in 50) as an infusion during the operation starting before skin incision.
-
Ketamine and dexmedetomidine were prepared in the same syringe (10 ml as bolus and 50 ml for infusion) with the following doses:
-
Ketamine: 0.5 mg/kg (IBW), dexmedetomidine: 1 mg/kg (TBW) as bolus to be administered over a 10-min period
-
The same dose used as bolus was added to a 50-ml syringe adjusted to a rate of 25 ml/h (ketamine 0.25 mg/kg/h (IBW) and dexmedetomidine 0.5 μg/kg/h (TBW)) as an infusion during the operation starting before skin incision.
-
-
-
Analgesic infusion and inhalation anaesthesia were stopped after skin closure.
-
Intra-operatively, Paracetamol 1 gm and diclofenac 75 mg (incorporated in IV infusion fluids) were given before emergence.
-
Patients were kept after extubation for observation in the PACU until fulfilling an Aldrete score of 9.
-
Post-operative analgesia was offered in regular doses of paracetamol 1 gm IV every 6 h for the following 48 h, and rescue doses of IV nalbuphine 5 mg if the VAS score is > 3.
-
Measured outcomes
The time between the end of analgesic use and extubation and the time between the end of analgesic use and an Aldrete score of 9. Postoperative pain score for 48 h using the visual analog scale (VAS), 30 min after recovery, hourly for 2 h and every 6 h for 48 h, and nalbuphine consumption during the 48 h following extubation. Postoperative hypoxemia (defined as peripheral oxygen saturation (SpO2) < 92% on room air). Number of episodes of PONV (during the 24 h following extubation). The number of bradycardia, hypotension, and hypertension events during surgery (bradycardia is defined as HR ≤ 50 bpm, hypotension is defined as a decrease in systolic blood pressure >20% of basal, hypertension is defined as an increase in systolic blood pressure >20% of basal).
Results
Data was analyzed using the statistical package for Social Science (SPSS) version 22.0. The data was tested for normality. The mean standard deviation (SD) was used to express quantitative data. Qualitative data was expressed as frequency and percentage.
The following tests were used:
-
An independent-samples t test was used when comparing quantitative data.
-
A chi-square (X2) test was used in order to compare qualitative parameters.
-
The confidence interval was set at 95% and the margin of error accepted was set at 5%. So, the P value was considered significant as the following:
-
Probability (P value) < 0.05 was considered significant.
Demographics
Sixty patients were enrolled in the study. Thirty patients in each group, TBA and OFA.
Groups were comparable in demographic data (in terms of age, sex, BMI, LBW) and duration of surgery (P value > 0.05) (Table 1).
Intraoperative vital data events in the form of hypotension, hypertension, and bradycardia were compared between the 2 groups (Table 2). The OFA group showed more hypotension events with a significant p value (p= 0.03)
.
Although OFA showed more bradycardia and fewer hypertension events, there was no statistical difference between the two groups as regards bradycardia and hypertension events.
The TBA group showed a statistically significant reduction in the time needed for extubation and reaching an Aldrete score of 9 (Table 3).
Data is expressed as mean ± SD, T = Student’s t test
The TBA group showed a higher incidence of PONV but was statistically insignificant (Table 4).
There was no significant difference in post-operative hypoxemia events between both groups (Table 5).
As regards postoperative nalbuphine consumption, the TBA group showed statistically significantly more nalbuphine consumption (P < 0.001) (Table 6).
The two groups were compared as regards pain control post operatively. A visual analog scale (VAS) was used to assess pain post operatively and was used at regular intervals (30 min, 1 h, 2 h, 6 h, 12 h,18 h, 24 h, 30 h, 36 h, 42 h, and 48 h) (Table 7).
After 30 min and 1 h, OFA showed significantly better pain control. Then, after 2 h, there was no statistical difference between them. After that, OFA showed better pain control again till 24 h. After that, for 48 h, there was no difference between the two groups.
Discussion
The patients enrolled in the current study were comparable in both groups as regards to the demographic data (age, sex, weight, and BMI). Most patients were females.
Intraoperative hemodynamic events showed no significant difference between the 2 groups except for hypotension in the OFA group. Hypotension events were not associated with bradycardia. This may be due to the definitions adopted by the current study for hypotension (20% decrease in BP from basal BP) and bradycardia (HR ≤ 50 bpm).
Both extubation time and the time patients reached an Aldrete score of 9 were longer in the OFA group.
There was no statistically significant difference between the two groups in post-operative hypoxemia events.
Although there was a decrease in the number of patients who experienced vomiting in the OFA group (5 patients) as compared to that in TBA (11 patients), this turned out to be statistically not significant.
There was a significant decrease in pain scores and nalbuphine consumption in the OFA group that extended to 24 h, except for at 2 h post-operative. Patients in the TBA group received a nalbuphine rescue dose in response to their elevated VAS score in the first hour. This may explain the similarity in VAS score at 2 h post-operative between both groups.
Many studies compared OFA with TBA in obese patients. They differed in the composition of the OFA regimen, the way of giving the drug (infusion or boluses), the outcomes they measured, and also the way they calculated the doses in obese people (based on IBW, LBW, or TBW).
Ibrahim et al. (2022) in their study that compared OFA in the form of a mixture of dexmedetomidine, lidocaine, and ketamine infusions with opioid based anesthesia (OBA) agreed with the current study that OFA showed more time to extubation and readiness to PACU discharge although dexmedetomidine was given according to IBW. Ketamine and fentanyl were also given according to IBW. Also, Beloeil et al. (2021) in a study that included different surgery types showed delayed extubation time and PACU stay in OFA in comparison to remifentanil where both groups received ketamine. This was not the case in the study done by El Sayed et al. (2017) that compared the use of dexmedetomidine infusion to placebo infusion (control group) with fentanyl boluses in both groups in response to hemodynamic changes, and the results showed a decrease in time to extubation and time to PACU discharge in the dexmedetomidine group. The total fentanyl consumption intra-operative was significantly higher in the control group than in the dexmedetomidine group, and fentanyl was given based on TBW, and not IBW, as in the current study, that may have led to opioid overdose and accounts for the longer time in the control group. Also, the maintenance dose of dexmedetomidine was slightly lower in the mentioned study than in the current study.
Ziemann-Gimmel et al. (2014) in their study of OFA accompanied by total intravenous anesthesia (TIVA) in comparison with OBA and inhalational anesthesia, they found no difference in time to extubation or time to PACU readiness to discharge between the two groups. Dexmedetomidine was given on TBW as in the current study, but both loading and maintenance doses were lower than (about half) of those used in the current study. Also, fentanyl was given based on TBW and not IBW.
Mulier et al. (2018) did not find a significant difference between OFA and OBA regarding intra operative hemodynamic events in bariatric surgeries. This may be explained by the fact that dexmedetomidine was given adjusted to IBW and even the loading dose was lower than that in the current study. In fact, the dexmedetomidine and ketamine loading doses used in the current study were half of what was used in the previous study. Elsaye et al. (2019) in their comparative study between OFA and OBA in laparoscopic cholecystectomy in morbid obese patients, they showed a difference between the two groups in the form of decreased HR and BP in the OFA group. Although dexmedetomidine was given at a lower dose than in the current study, lidocaine and magnesium sulphate were given both for loading and maintenance based on TBW. This may explain the augmented effect on HR and BP intra-operatively. Beloeil et al. (2021) study showed more bradycardia events in the dexmedetomidine group, which may be attributed to the high dose adopted by the study at first before modification.
Aronsohn et al. (2021) did not find a significant difference between OFA and OBA as regards to pain intensity and post-operative opioid consumption. The study regimen was either dexmedetomidine, ketamine, or lidocaine, dexmedetomidine was not given as a loading dose, and the maintenance dose was lower than that of the current study (0.2–0.6 μg/kg/hr). Also, a meta-analysis study for randomized controlled trials for OFA by Salomé et al. (2021) found no significant difference between OFA and OBA in terms of pain and opioid consumption post-operative. The trials included did not share the same drugs, doses, or combinations of drugs.
Mulier et al. (2018) found that patients receiving OFA require fewer analgesics, have a better quality of recovery after surgery, have a better VAS score, and require fewer opioids in the PACU and ward, despite the fact that the doses used were lower than those used in the current study. Also, Ibrahim et al. (2022) study showed a reduction in pain scores in PACU and till 6 h postoperatively in the OFA group. Toleska and Dimitrovski (2019), Mansour et al. (2013), Abdelmoniem et al. (2020), and Beloeil et al. (2021) also reported that patients who received fentatnyl showed higher pain scores in comparison to opioid free anesthesia.
As regards to post-operative nausea and vomiting, most studies showed a significant reduction in OFA, as Salomé et al. (2021), Ziemann-Gimmel et al. (2014), Mulier et al. (2018), Abdelmoniem et al. (2020), and Beloeil et al. (2021), while Aronsohn et al. (2021) study and Mansour et al. (2013) showed no difference.
Nalbuphine, the rescue medication used, is a mixed agonist antagonist, and some may argue that its use after fentanyl in the postoperative period may result in the reversal of analgesia, that could be the reason for the higher pain scores in the TBA group, but nalbuphine antagonistic action on pure opioid agonists was examined before.
The study done by Gunion et al. (2004) reported that nalbuphine and other opioids can be used concurrently with better analgesia and fewer side effects as nalbuphine has the potential to attenuate the mu-opioid effects and to enhance the kappa-opioid effects. Also, Davis et al. (2018) reported that low doses of nalbuphine when combined with potent opioids reduces side effects, particularly respiratory depression without loss of analgesia.
Moreover, Yeh et al. (2008) examined mixing nalbuphine and morphine together in PCA in different ratios and concluded that the interaction between morphine and nalbuphine in PCA admixture on analgesia is additive.
Also, Seol et al. (2003) examined mixing nalbuphine and fentanyl in PCA and concluded that nalbuphine with fentanyl in combination is a useful method for intravenous PCA.
Back to the current study, the results showed that the VAS score for both groups at 2 h after operation was equal and the authors attributed that to the fact that the TBA group showed a high vas score at 1 h post-operative and received nalbuphine as a rescue medication.
Limitations
The current study did not use the BIS module or Train Of Four (TOF) intra-operative. This may have played a role in intraoperatively adjusting inhalational anesthesia, which may have affected the outcome and standardized the extubation process.
Conclusions
OFA has a better profile than TBA with regard to post-operative pain score and post-operative opioid consumption, but they have a relative increase in time to extubation and time to reach an Aldrete score of 9.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- ERAS:
-
Enhanced recovery after surgery
- HR:
-
Heart rate
- IBW:
-
Ideal body weight
- IV:
-
Intravenous
- LBW:
-
Lean body weight
- OBA:
-
Opioid based anesthesia
- OFA:
-
Opioid free anesthesia
- PACU:
-
Post-anesthesia care unit
- PONV:
-
Post-operative nausea and vomiting
- TBA:
-
Traditional balanced anesthesia
- TBW:
-
Total body weight
- VAS:
-
Visual analog scale
References
Abdelmoniem M, Farran H, Soliman S, Alfy M (2020) Opioid free anesthesia in patients undergoing three-ports laparoscopic cholecystectomy. Al-Azhar Int Med J. 1(2):160–165. https://doi.org/10.21608/aimj.2020.22111.1059
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA et al (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 342(18):1301–1308. https://doi.org/10.1056/NEJM200005043421801
Aronsohn J, Zeltsman M, Gerasimov M, Palleschi G, Yang P (2021) Opioid-Free an-esthesia in bariatric surgery: a retrospective cohort study. Int J Anesthetic Anesthesiol. 8:120
Beloeil H, Garot M, Lebuffe G, Gerbaud A, Bila J, Cuvillon P, Dubout E, Oger S, Nadaud J, Becret A, Coullier N, Lecoeur S, Fayon J, Godet T, Mazerolles M, Atallah F, Sigaut S, Choinier PM, Asehnoune K, Roquilly A, Chanques G, Esvan M, Futier E, Laviolle B, POFA Study Group, SFAR Research Network (2021) Balanced opioid-free anesthesia with dexmedetomidine versus balanced anesthesia with remifentanil for major or intermediate noncardiac surgery the postoperative and opioid-free anesthesia (POFA) randomized clinical trial. Anesthesiology. 4(4):541–551. https://doi.org/10.1097/ALN.0000000000003725
Brown EN, Pavone KJ, Naranjo M (2018) Multimodal general anesthesia: Theory and practice. Anesth Analg. 127(5):1246–1258. https://doi.org/10.1213/ANE.0000000000003668
Davis MP, Fernandez C, Regel S, McPherson ML (2018) Does nalbuphine have a niche in managing pain? J Opioid Manag. 14(2):143–151. https://doi.org/10.5055/jom.2018.0441
Echeverria-Villalobos M, Stoicea N, Todeschini AB, Fiorda-Diaz J, Uribe AA, Weaver T, Bergese SD (2020) Enhanced Recovery after Surgery (ERAS): a perspective review of postoperative pain management under ERAS pathways and its role on opioid crisis in the United States. Clin J Pain. 36(3):219–226. https://doi.org/10.1097/AJP.0000000000000792
Egan TD (2019) Are opioids indispensable for general anaesthesia? Br J Anaesth 122(6):e127–e135 Available from: https://doi.org/10.1016/j.bja.2019.02.018
El Sayed M, Abdelsamad A, Amer A (2017) Shorter postanesthesia care unit stay with dexmedetomidine infusion during laparoscopic bariatric surgery: a randomized controlled trial. Res Opin Anesth Intensive Care. 4(3):129. https://doi.org/10.4103/roaic.roaic_103_16
Elsaye R, Gaafary AM, Elsaeid A (2019) Comparative study between the effect of opioid-free anesthesia versus opioid-based anesthesia in morbid obese patients. Sci J Al-Azhar Med Fac Girls 3(2):457
Greenshields N, Mythen M (2020) Enhanced recovery after surgery. Curr Anesthesiol Rep. 10(1):49–55. https://doi.org/10.1007/s40140-020-00372-y
Gunion MK, Marchionine AM, Anderson CTM (2004) Use of the mixed agonist – antagonist nalbuphine in opioid based analgesia. Acute pain 6(1):29–39. https://doi.org/10.1016/j.acpain.2004.02.002
Gupta K, Nagappa M, Prasad A, Abrahamyan L, Wong J, Weingarten TN, Chung F (2018) Risk factors for opioid-induced respiratory depression in surgical patients: a systematic review and meta-analyses. BMJ Open. 8(12):1–10. https://doi.org/10.1136/bmjopen-2018-024086
Gupta S, Mohta A, Gottumukkala V (2020) Opioid-free anesthesia — caution for a one- size-fits-all approach. Perioper Med. 9(1):16–19. https://doi.org/10.1186/s13741-020-00147-3
Ibrahim M, Elnabtity AM, Hegab A, Alnujaidi OA, El Sanea O (2022) Combined opioid free and loco-regional anesthesia enhances the quality of recovery in sleeve gastrectomy done under ERAS protocol: a randomized controlled trial. BMC Anesthesiol 22(1):29
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B (2005) Quantification of lean bodyweight. Clin Pharmacokinet. 44(10):1051–1065. https://doi.org/10.2165/00003088-200544100-00004
Mansour MA, Mahmoud AAA, Geddawy M (2013) Nonopioid versus opioid based general anesthesia technique for bariatric surgery: a randomized double-blind study. Saudi J Anaesth. 7(4):387–391. https://doi.org/10.4103/1658-354X.121045
Mulier JP, Wouters R, Dillemans B, Dekock M (2018) A randomized controlled, double-blind trial evaluating the effect of opioid-free versus opioid general anaesthesia on postoperative pain and discomfort measured by the QoR-40. J Clin Anesth Pain Med. 2(1):1–6
Ortiz VE, Kwo J (2015) Obesity: physiologic changes and implications for preoperative management. BMC Anesthesiol. 15(1):1–12
Salomé A, Harkouk H, Fletcher D, Martinez V (2021) Opioid-free anesthesia benefit–risk balance: a systematic review and meta-analysis of randomized controlled trials. J Clin Med 10(10):2069
Samuels D, Abou-samra A, Dalvi P, Mangar D, Camporesi EM (2017) Opioid-free anesthesia results in reduced post-operative opioid consumption. J Clin Anesth Pain Med. 1(2):2–4
Seol GY, Choi JS, Park CH, Lee CS, Kim WT (2003) A Comparison of the effect of fentanyl and fentanyl-nalbuphine for postoperative analgesia using IV-PCA. Korean J Anesthesiol 45:481. https://doi.org/10.4097/kjae.2003.45.4.481
Soffin EM, Wetmore DS, Beckman JD, Sheha ED, Vaishnav AS, Albert TJ, Gang CH, Qureshi SA (2019) Opioid-free anesthesia within an enhanced recovery after surgery pathway for minimally invasive lumbar spine surgery: a retrospective matched cohort study. Neurosurg Focus. 46(4):1–9. https://doi.org/10.3171/2019.1.FOCUS18645
Thota RS, Ramkiran S, Garg R, Goswami J, Baxi V, Thomas M (2019) Opioid free onco-anesthesia: Is it time to convict opioids? A systematic review of literature. J Anaesthesiol Clin Pharmacol. 35(4):441–452. https://doi.org/10.4103/joacp.JOACP_128_19
Toleska M, Dimitrovski A (2019) Is opioid-free general anesthesia more superior for postoperative pain versus opioid general anesthesia in laparoscopic cholecystectomy? Prilozi. 40(2):81–87. https://doi.org/10.2478/prilozi-2019-0018
Yeh YC, Lin TF, Lin FS, Wang YP, Lin CJ, Sun WZ (2008) Combination of opioid agonist and agonist-antagonist: patient-controlled analgesia requirement and adverse events among different-ratio morphine and nalbuphine admixtures for postoperative pain. Br J Anaesth. 101(4):542–548. https://doi.org/10.1093/bja/aen213
Ziemann-Gimmel P, Goldfarb AA, Koppman J, Marema RT (2014) Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. Br J Anaesth. 112(5):906–911. https://doi.org/10.1093/bja/aet551
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
AM revised the literature, collected the data, followed-up the patients, and wrote the manuscript. RA designed the study, performed the analysis, and wrote and critically revised the manuscript. MAE revised the literature, performed the analysis, and critically reviewed the manuscript. IM revised the literature, collected the data, and critically reviewed the manuscript. MAA designed the study, revised the literature and shared in writing the manuscript. The authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
After approval of the ethical committee in Faculty of Medicine, Ain Shams University number FMASU M D 182/2019, this interventional, randomized, and double–blinded controlled trial was conducted in Ain Shams University Hospitals. An informed written consent was taken from every patient one day before the surgery.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soudi, A.M., Hammad, R.A., ElShafie, M.A. et al. Comparing opioid free general anesthesia to traditional balanced general anesthesia regarding achievement of enhanced recovery in laparoscopic bariatric surgeries. Ain-Shams J Anesthesiol 14, 24 (2022). https://doi.org/10.1186/s42077-022-00218-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-022-00218-1