- Research
- Open access
- Published:
Lipidomic studies revealing serological markers associated with the occurrence of retinopathy in type 2 diabetes
Journal of Translational Medicine volume 22, Article number: 448 (2024)
Abstract
Purpose
The duration of type 2 diabetes mellitus (T2DM) and blood glucose levels have a significant impact on the development of T2DM complications. However, currently known risk factors are not good predictors of the onset or progression of diabetic retinopathy (DR). Therefore, we aimed to investigate the differences in the serum lipid composition in patients with T2DM, without and with DR, and search for potential serological indicators associated with the development of DR.
Methods
A total of 622 patients with T2DM hospitalized in the Department of Endocrinology of the First Affiliated Hospital of Xi’an JiaoTong University were selected as the discovery set. One-to-one case–control matching was performed according to the traditional risk factors for DR (i.e., age, duration of diabetes, HbA1c level, and hypertension). All cases with comorbid chronic kidney disease were excluded to eliminate confounding factors. A total of 42 pairs were successfully matched. T2DM patients with DR (DR group) were the case group, and T2DM patients without DR (NDR group) served as control subjects. Ultra-performance liquid chromatography–mass spectrometry (LC–MS/MS) was used for untargeted lipidomics analysis on serum, and a partial least squares discriminant analysis (PLS-DA) model was established to screen differential lipid molecules based on variable importance in the projection (VIP) > 1. An additional 531 T2DM patients were selected as the validation set. Next, 1:1 propensity score matching (PSM) was performed for the traditional risk factors for DR, and a combined 95 pairings in the NDR and DR groups were successfully matched. The screened differential lipid molecules were validated by multiple reaction monitoring (MRM) quantification based on mass spectrometry.
Results
The discovery set showed no differences in traditional risk factors associated with the development of DR (i.e., age, disease duration, HbA1c, blood pressure, and glomerular filtration rate). In the DR group compared with the NDR group, the levels of three ceramides (Cer) and seven sphingomyelins (SM) were significantly lower, and one phosphatidylcholine (PC), two lysophosphatidylcholines (LPC), and two SMs were significantly higher. Furthermore, evaluation of these 15 differential lipid molecules in the validation sample set showed that three Cer and SM(d18:1/24:1) molecules were substantially lower in the DR group. After excluding other confounding factors (e.g., sex, BMI, lipid-lowering drug therapy, and lipid levels), multifactorial logistic regression analysis revealed that a lower abundance of two ceramides, i.e., Cer(d18:0/22:0) and Cer(d18:0/24:0), was an independent risk factor for the occurrence of DR in T2DM patients.
Conclusion
Disturbances in lipid metabolism are closely associated with the occurrence of DR in patients with T2DM, especially in ceramides. Our study revealed for the first time that Cer(d18:0/22:0) and Cer(d18:0/24:0) might be potential serological markers for the diagnosis of DR occurrence in T2DM patients, providing new ideas for the early diagnosis of DR.
Introduction
Type 2 diabetes mellitus (T2DM) is a common chronic disease in many countries, and its prevalence is growing as people’s lifestyles are changing [1]. Diabetes causes various complications, classified as either macrovascular complications (such as cardiovascular disease and stroke) or microvascular complications (such as kidney disease) [2]. Diabetic retinopathy (DR), a specific microvascular complication of diabetes, is the most common cause of vision loss in people of working age [3, 4]. Poor glycemic control, hypertension, and diabetes duration are major risk factors for DR [5]. Although intensive risk factor control reduces the risk of DR progression and vision loss, many diabetic patients continue to develop DR with strict glycemic and blood pressure control [6]. Despite increasing research supporting the efficacy of routine DR screening to prevent DR and early treatment to reduce the risk of vision loss, there are no specific biomarkers for diagnosing the onset and early progression of DR. Additionally, new and more effective strategies are awaited to prevent and treat the progression of DR.
Accumulating evidence suggests that disruption in lipid metabolism is an early event in the pathogenesis of diabetes complications. Previous studies found that levels of multiple lipid species, including glycerophospholipids, sphingolipids and glycerolipids, are critical risk factors for T2DM and its complications [7, 8]. Lysophosphatidylcholine (LPC) is a main glycerophospholipid known for its essential role in lipid and glucose metabolism, and LPC has been intensively studied in the development of metabolic diseases including T2DM [9]. Sphingolipids, including ceramides (Cer), sphingomyelins (SM) and gangliosides, have a variety of intra- and extracellular effects on glucose homeostasis and metabolic disease [10] Numerous studies suggest Cer, a crucial lipid intermediate in sphingolipid metabolism, is a major contributing factor for insulin resistance, and inhibition or depletion of enzymes driving de novo ceramide synthesis can prevent the development of diabetes in mice [7, 11, 12]. In contrast, a decrease in very long chain Cer is correlated with the development of macroalbuminuria in diabetes [13]. Accelerated sphingolipid catabolism’ leading to an increase in glucosylceramide or glycosphingolipids might contribute to the neuronal pathologies of DR [14]. In addition, SM produced by the transfer of a phosphocholine moiety from phosphatidylcholine to the ceramide backbone has been linked to insulin resistance [15, 16] and is also an independent marker of cardiovascular disease [17]. Thus, dysregulated lipid metabolism is a major contributor to the pathogenesis of T2DM and its complications, and specific lipid species that are responsible for the occurrence of DR are rather obscure.
Lipidomics offers solid platforms for identifying novel lipid mediates in biochemical processes of lipid metabolism, thus providing new opportunities for disease prediction and detection [18, 19]. Lipidome analysis is performed by liquid chromatography and electrospray ionization-tandem mass spectrometry (LC–MS/MS) for molecular lipid identification and quantification and multiple reaction monitoring (MRM) for targeted quantification of those lipid species. Lipid-based biomarkers offer unique options for precision medicine by providing sensitive diagnostic tools for disease prediction and monitoring [20]. Using a quantitative metabolomics approach, Emil et al. compared the aqueous humor and serum concentrations of metabolites in senior adults with an without diabetes who underwent cataract surgery [21]. However, the field of lipidomics studies of DR is still in its early stages, with few studies published and little replication of results [22].
In this study, we aimed to find reliable serum lipid-based biomarkers for the presence of DR in patients with T2DM by using two cohorts. To this end, serum samples of the discovery cohort was subjected to untargeted lipidomics analysis to search for differentially abundant lipids between individuals without and with DR. In the validation cohort, the observed differential lipid molecules were validated using mass spectrometry MRM targeting techniques. We hypothesized that DR has a distinctive serum lipid signature and that particular lipid species can act as biomarkers for T2DM patients with DR.
Research design and methods
Participants
A total of 622 participants with T2DM hospitalized in the Endocrinology Department of the First Affiliated Hospital of Xi’an JiaoTong University were screened as the discovery set. Participants with chronic kidney disease [estimated glomerular filtration rate (eGFR) < 90 (mL/min/1.73 m2)] were excluded from the selection. We conducted pair matching according to the traditional risk factors for DR (including age, duration of diabetes, HbA1c level, and hypertension). For the discovery cohort, we selected 42 T2DM patients with DR (DR group). The control participants were 42 T2DM patients without DR (NDR group), and they were matched to patients in the DR group by age (in 5-year bands), diabetes duration (in 5-year bands), HbA1c levels (in 0.5% bands), and hypertension status.
Lipid markers of DR identified from the discovery cohort were quantified in a separate sample cohort (validation cohort). We first screened 531 T2DM patients. Individuals with chronic kidney disease [eGFR < 90 (mL/min/1.73 m2)] were excluded from the selection. Then, we conducted 1:1 propensity score matching (PSM) (matching tolerance = 0.02) by age, diabetes duration, HbA1c level, hypertension status, sex, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), and eGFR. For the validation cohort, 95 T2DM patients with DR (DR group) and 95 T2DM patients without DR (NDR group) were included.
Sample collection
Fasting blood samples and clinical data were collected from the individuals. All blood samples were collected at the First Affiliated Hospital of Xi’an JiaoTong University physical examination center. Blood samples were centrifuged for 20 min at 1500 rpm and 4 °C. Then, serum was collected and stored at -80 °C until analysis. HbA1c was measured using an automatic HbA1c analyzer (TOSOH BIOSCIENCE, INC.; HLC-723G8). Total cholesterol (CHOL), triglyceride (TG), high density lipoprotein-cholesterol (HDL-c), low density lipoprotein-cholesterol (LDL-c), uric acid (UA), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), total bilirubin (TBIL), direct bilirubin (DBIL), total protein (TP), albumin (ALB), glucose (GLU), blood urea nitrogen (BUN), creatinine (CRE) were measured using standard reagents on an automatic biochemistry analyzer (HITACHI, Inc.; LAbOSPECT, 008AS). Blood pressure was measured in triplicate using an Omron HBP-9020 digital automatic blood pressure machine (Kyoto, Japan).
Lipid extraction
The serum samples were thawed slowly at 4 °C, 100 µL of the sample was placed in a 96-well plate, 300 µL of isopropanol (prechilled at -20 °C) spiked with internal standards (SPLASH® LIPIDOMIX® Mass Spec Standard, Avanti, USA) was added, and the samples were vortexed and mixed for 1 min and then centrifuged at 4 °C for 20 min at 4000 rcf after resting overnight at -20 °C as previously reported [23]. The supernatant was injected for LC–MS/MS analysis, and 10 µL of each supernatant was mixed into quality control (QC) samples to assess the reproducibility and stability of the LC–MS analysis process.
LC–MS/MS analysis
Lipids were separated and detected by an UPLC (CSH C18 column, 1.7 μm 2.1*100 mm, Waters, USA) equipped with a Q Exactive Plus high-resolution mass spectrometer (Thermo Fisher Scientific, USA) as previously reported [24]. The following gradient was used for elution: 0–2 min, 40-43% mobile phase B (10 mM ammonia formate, 0.1% formic acid, 90% isopropyl alcohol, and 10% acetonitrile); 2–2.1 min, 43-50% liquid B; 2.1–7 min, 50-54% solution B; 7–7.1 min, 54-70% liquid B; 7.1–13 min, 70-99% liquid B with a flow rate of 0.35 mL/min. Mobile phase A was an aqueous solution containing 10 mM ammonia formate, 0.1% formic acid and 60% acetonitrile in water.
All samples were analyzed in data-dependent acquisition (DDA) mode with the following positive/negative ionization settings: spray voltage, 3.8/–3.2 kV; aux gas heater temperature, 350 °C; and capillary temperature, 320 °C. The full scan mass range was 200–2000 m/z with 70,000 mass resolution at m/z 200 and AGC set to 3e6 with a maximum ion injection time of 100 ms. The top three precursors were selected for subsequent MS fragmentation with a maximum ion injection time of 50 ms and resolution of 17,500 at m/z 200, and the AGC was 1e5. The stepped normalized collision energy was set to 15, 30, and 45 eV.
Data preprocessing and quality control
The raw data obtained from the LC–MS/MS detection were imported into LipidSearch v.4.1 (Thermo Fisher Scientific, USA) for lipid identification and quantification. The following parameters were used for lipid identification and peak extraction: the type of identification was Product, the mass deviation of the parent and daughter ions was 5 ppm, and the response threshold was set to 5.0% of the relative response deviation of the daughter ions; the quantitative parameters were set to calculate the peak areas of all identified lipids, and the peak extraction mass deviation was set to 5 ppm. For ESI + data, [M + H]+, [M + NH4]+, and [M + Na] + were selected as adducts, while for ESI- data, [M-H]-, [M-2 H]-, and [M-HCOO]- were selected as adducts. The peak alignment was performed for all identified lipids, and those not marked as “rejected” were considered for inclusion in the subsequent analysis.
For data preprocessing, raw data exported from LipidSearch were further analyzed by meta X [25]. The data preprocessing included (1) Removing lipid molecules with more than 50% missing information in QC samples and more than 80% missing information in experimental samples (i.e., LipidIon in the table); (2) Filling the missing values using the k-nearest neighbor (KNN) algorithm; (3) Correcting the batch effect using quality control-based robust LOESS signal correction (QC-RLSC); (4) Using probabilistic quotient normalization (PQN) to normalize the data to obtain the relative peak areas; and (5) Removing the lipid molecules with a coefficient of variation (CV) greater than 30% of the relative peak areas from all QC samples.
Data quality was assessed by the reproducibility of QC sample assays. The assessment included chromatogram overlap of QC samples, principal component analysis (PCA), number of extracted peaks, and differences in peak response intensity.
Data processing
A combination of multivariate statistical analysis and univariate analysis was used to screen for lipids of which the abundance differed between groups. The multivariate statistical analysis methods used were principal component analysis (PCA) and partial least squares method-discriminant analysis (PLS-DA). PCA is an unsupervised pattern recognition method, and PLS-DA is a supervised pattern recognition method. The univariate analyses were fold change (FC) and Student’s t test. The FC was obtained by fold change analysis, and the p value pairs of the t test were corrected for the false discovery rate (FDR) to obtain a q-value. The differential lipid molecule screening conditions were as follows: (1) variable importance in the projection (VIP) ≥ 1 for the first two principal components of the PLS-DA model; (2) fold change ≥ 1.2 or ≤ 0.83; and (3) p value < 0.05.
Targeted lipid quantification by MRM in validation samples
The identified differential lipids were further quantified by multiple reaction monitoring (MRM). For lipid extraction, the procedure was consistent with the untargeted experiment as described. The MRM transition list is shown in Table S1. For MRM quantification, all validation samples were analyzed on a QTRAP 5500 mass spectrometer with a CSH C18 column (1.7 μm 2.1*100 mm, Waters, USA) for separation. All lipids were subjected to targeted quantification in ESI + mode with a specific transition setting.
Statistical analysis
The clinical data of samples are presented as the mean ± standard deviation (SD) for normally distributed variables or the median (interquartile range) for abnormal distribution. Comparisons between the case group and the control group were made using a two-tailed t test or Mann-Whitney U test for continuous data and the X2 test for categorical data. The calculation of the area under the curve (AUC) in receiver operating characteristic (ROC) curve analysis was used to evaluate the discriminatory ability of the markers. Logistic regression models were applied to assess the relationship between lipid molecules and the presence of DR. The odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for the molecules with 1-SD changes. The known risk factors for DR, such as CHOL, TGs, LDL-c, and HDL-c, were added to multivariate logistic regression to calculate the adjusted odds ratios. Ordinal logistic regression models were used to assess the relationships between lipid molecules and DR stages [NDR, nonproliferative DR (NPDR) and proliferative DR (PDR)].
Results
Characteristics of the discovery cohort
Table 1 shows the clinical characteristics of individuals selected for the discovery cohort. There were no significant differences in age and sex between the DR and NDR groups. In fact, these groups were comparable for most metabolic characteristics, such as BMI, diabetes duration, and HbA1c, and there were no significant between-group differences for hypertension status, antihypertensive agent use, hypoglycemic therapy status or NSAID use. The blood pressure and glucose of the participants were treated and controlled. Compared with control subjects with T2DM, T2DM patients with DR had higher levels of LDL-c levels, AST, TBIL, and BUN (Table 1 and Table S2).
Untargeted lipidome-derived biomarkers for diabetic retinopathy: results from the discovery cohort
A total of 1721 lipids were detected. The number of lipids with an RSD (CV) less than or equal to 30% in the QC samples was 1421. The ratio of the number of lipids with CV less than or equal to 30% to the number of all detected lipids in QC samples was 81%.
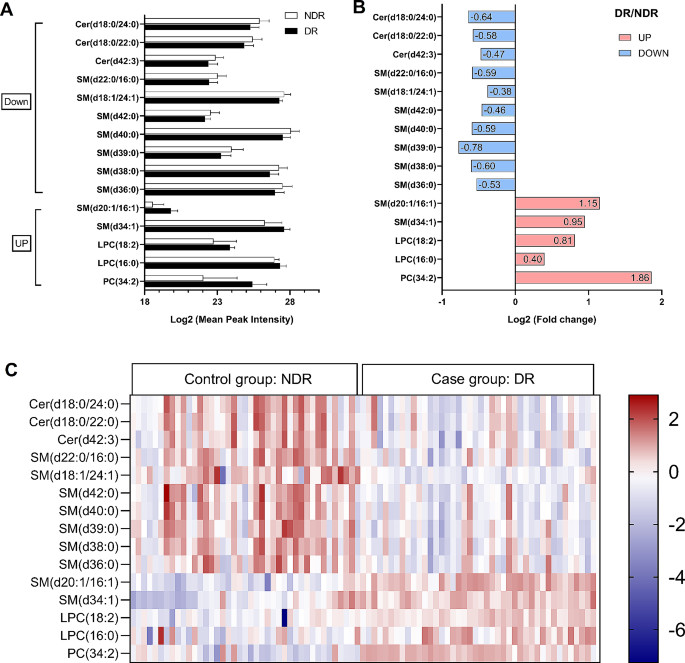
Fifteen candidate lipids were identified from the discovery cohort. Compared with those of the NDR group, the levels of three Cer and seven SM were significantly lower in the DR group. In contrast, two SM, two LPC and one PC were significantly higher in the DR group (Fig. 1A and B). More specifically, compared with T2DM patients without DR, T2DM patients with DR showed lower levels of Cer(d18:0/24:0), Cer(d18:0/22:0), Cer(d42:3), SM(d22:0/16:0), SM(d18:1/24:1), SM(d42:0), SM(d40:0), SM(d39:0), SM(d38:0), and SM(d36:0), and higher levels of SM(d20:1/16:1), SM(d34:1), LPC(18:2), LPC(16:0) and PC(34:2). The heat map shows the distribution of these lipids between individuals of the NDR and DR groups (Fig. 1C). The results of ROC analysis and the odds ratios of the lipid markers in the basic logistic regression models are shown in Table 2. The AUC values for the 15 lipids ranged from 0.72 to 0.94. All lipids retained significant ORs after adjusted for CHOL, TG, LDL-c, and HDL-c (adjusted ORs are shown in Table 2). Furthermore, we used ordinal logistic regression, which estimated the odds of being in one higher category of the DR stage (from NDR to PDR) for lipid species, to test the associations between lipid species and DR stage (Table S3; n = 42 in the NDR group, n = 37 in the NPDR group, n = 5 in the PDR group), and we analyzed the data while excluding participants with diabetic macular edema (DME) (n = 4 in the DR group), as before, all lipids retained significant ORs (Table S4).
Lipidome-derived markers identified from the discovery cohort. Lipidomic analysis identified fifteen candidate lipids of which serum levels were different between 42 T2DM patients with DR (DR group) and 42 T2DM patients without DR (NDR group) from the discovery cohort. (A) Mean peak intensity of lipids was analyzed after Log2 transformation of the data. (B) Fold change in DR/NDR was analyzed after Log2 transformation of the data. (C) Heatmap showing the distribution of lipid markers. Each row in the figure represents a different lipid, and each column represents a sample. Different colors indicate different intensities, and Log2 conversion was used for the data
Characteristics of the validation cohort and targeted lipidomics analysis
The 15 differential lipids found from the discovery cohort were validated in another set of samples. The clinical characteristics of individuals selected for the validation cohort are shown in Table 3. Most metabolic and clinical features were comparable (Table S5), and there was no significant difference in LDL-c between the DR and NDR groups.
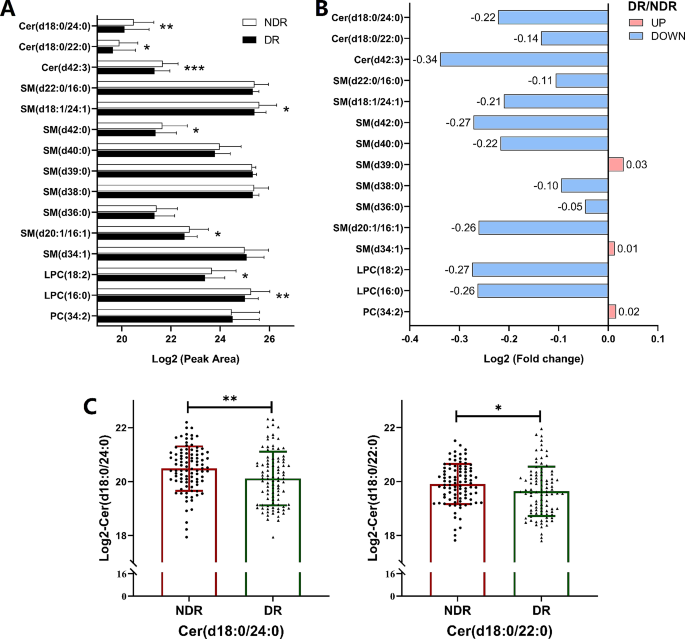
In the validation cohort, when compared with subjects in the NDR group, T2DM patients with DR showed lower levels of Cer(d18:0/24:0), Cer(d18:0/22:0), Cer(d42:3) and SM(d18:1/24:1) by univariate logistic regression, which was consistent with the results of the discovery cohort. However, the levels of SM(d20:1/16:1), LPC(18:2) and LPC(16:0) were lower in T2DM patients with DR from the validation cohort, opposite to the result obtained in the discovery cohort (Fig. 2A and B). The AUC values for these lipids were higher than 0.61. The other 8 lipids did not significantly differ between the DR and NDR groups in the validation cohort (Table 4). Of note, compared with those in T2DM patients, the peak area (after Log2 transformation) of Cer(d18:0/24:0) (20.48 ± 0.82 vs. 20.12 ± 0.99, p = 0.006, Fig. 2C) and Cer(d18:0/22:0) (19.91 ± 0.75 vs. 19.64 ± 0.92, p = 0.028, Fig. 2C) remained significantly lower in T2DM patients with DR, and the levels of these two lipids retained significant ORs when adjusted for known risk factors (i.e., CHOL, TG, LDL-c and HDL-c). In the ordinal regression, these two lipids maintained significant ORs (Table S7, n = 95 in the NDR group, n = 87 in the NPDR group; n = 8 in the PDR group), and were also significant while excluding patients with DME (Table S6, n = 2 in the DR group). These findings imply that levels of Cer(d18:0/24:0) and Cer(d18:0/22:0) were independent markers for T2DM patients with DR in both the discovery cohort (Table 2) and validation cohort (Table 4).
The results of targeted lipidomics analysis in the validation cohort. For the validation cohort, the cases were 95 T2DM patients with DR (DR group), and the control subjects were 95 T2DM patients who had no DR (NDR group). (A) Peak area of lipids was analyzed after Log2 transformation of the data. (B) Fold change in DR/NDR was analyzed after Log2 transformation of the data. (C) The log2 conversion was used for the intensities of Cer(d18:0/24:0) and Cer(d18:0/22:0). All data are presented as the mean ± standard deviation (SD). Each symbol represents an individual participant. *p < 0.05, **p < 0.01, pairwise comparisons of change scores between the groups were evaluated by t test
Discussion
DR is the most common microvascular complication of diabetes and the main factor contributing to visual impairment in working-age individuals [3]. T2DM patients often develop DR despite of proper control of systemic risk factors, indicating the involvement of other pathogenic factors for DR development. To find new and more effective strategies for preventing and treating DR, it is necessary for us to identify novel biomarkers for DR screening or detection. Lipidomics will aid in understanding the mechanism of DR at various stages of the disease, early diagnosis, and the identification of new therapeutic targets. In this study, by using two clinical cohorts, we found that the serum lipidomic profiles in T2DM patients with DR showed significant differences from those in T2DM patients without DR. The differential lipid species in the DR group were linked to disturbances in sphingolipid metabolism. Compared with those in the NDR group, the levels of Cer(d18:0/24:0) and Cer(d18:0/22:0) were significantly lower in the DR group after adjusting for covariates, i.e. known risk factors in both the discovery and validation cohorts. These findings suggest that these two lipid species may be potential serological markers for the diagnosis of DR in patients with T2DM.
In this study, we found two ceramide molecules that were significantly lower in T2DM patients with DR, indicating that they may have disturbed ceramide metabolism compared to T2DM patients without DR. Ceramide is sphingolipid [11] and can be found in VLDL, LDL, and HDL. Consistent with our findings, Fort et al. found a significantly lower abundance of Cer in central retinal tissue obtained postmortem from T2DM patients with DR compared to those without DR [26]. Similarly, ceramide levels were shown to be lower and glucosylceramide levels higher in the retinas of diabetic rodents [27]. This indicates that diabetes reduces the retinal ceramide content and may suggest that dysregulated sphingolipid metabolism may cause retinal resistance to insulin action [27]. These findings imply that ceramide is diverted from the overall pools of retinal sphingolipids toward the glycosylated forms due to hyperglycemia. In contrast, Levitsky et al. found that diabetes-induced increases in mitochondrial ceramide led to impaired mitochondrial function in the retinal pigment epithelial (RPE) cells of the retina [28], and disruption of the blood-retinal barrier might be caused by diabetes-induced overexpression of acid sphingomyelinase. Additionally, inflammation is a common underlying factor in DR, and inflammation generates Cer from SM in the serum membrane. This induces death receptor ligand formation and leads to apoptosis of RPE and photoreceptor cells [29]. In addition to diabetes, circulating Cer was shown to strongly correlate with future adverse cardiovascular events. It has recently been discovered that in individuals with atherosclerotic CVD, serum levels of specific Cer species can predict the future risk of cardiovascular death. In the Corogene study, higher concentrations of Cer(d18:1/16:0), Cer(d18:1/18:0), and Cer(d18:1/24:1) and lower concentrations of Cer(d18:1/24:0) were associated with a higher risk of fatal myocardial infarction [30]. Our study found that Cer(d18:0/24:0) and Cer(d18:0/22:0) were significantly lower in T2DM patients with DR compared to those without DR, which suggests that different numbers of carbons and double bonds in ceramides might play differential roles in DR and CVD. The distinct ceramides and ceramide metabolites involved in metabolic regulation play unanticipated roles [31]. Watt et al. discovered that circulating ceramides present in LDL particles were sufficient to induce insulin resistance in vitro and in vivo [32]. However, how these two identified ceramides influence lipid metabolism in T2DM remains unclear and needs further exploration. Thus, disturbed Cer metabolism may contribute to dysfunction in DR, and therapeutic strategies to restore normal Cer metabolism might be an effective approach for treatment of DR.
In the discovery cohort, LPC(18:2) and LPC(16:0) were significantly higher in T2DM patients with DR. However, these two lipids were significantly lower in DR in the validation cohort. The previous findings point to a change in sphingolipid composition between control and T2DM [33]. LPC is an inflammatory phospholipid and an important atherogenic substance in LDL that contributes to diabetic complications [34]. Lipoprotein-associated phospholipase A2 (Lp-PLA2) plays a crucial role in diabetes-related retinal vasopermeability, a response mediated by LPC, and inhibiting Lp-PLA2 reduces diabetes-induced retinal vasopermeability [35]. LPC O-16:0, LPC P-16:0, LPC O-18:0, and LPC 18:1 were all found to be inversely related to incident T2DM [36]. The differences between the discovery and validation cohorts may be related to the populations studied, medications used, and stages of diabetic retinopathy [37].
There are some limitations of this study. First, only a Chinese ethnic group was selected, and future validation of our findings in other races or ethnic groups is warranted. Second, instead of chronic risk factors associated with the development of DR, some of the identified lipid markers might only represent temporary metabolic perturbations in this cross-sectional study. Third, the exact mechanism of DR development in patients with T2DM through which ceramide functions has not been explained. Therefore, more extensive preclinical and clinical studies are needed to clarify the mechanisms behind the potential effects of specific lipids.
Overall, the deregulation of sphingolipid metabolism in the diabetic retina appears to be a significant and seldom-studied element of DR pathophysiology. The precise mechanism underlying this disease is still unknown and requires further investigation. We showed the potential value of lipidomics research in understanding the pathophysiology of DR, and the results suggest that lipidomics profiling may be capable of identifying early-stage DR diagnostic indicators in high-risk Chinese populations. In addition, the findings from this study may help in the elucidation of new therapeutic targets for DR prevention and treatment.
Data availability
All relevant data and materials have been included in the article and its supplementary data files. Further inquiries can be directed to the corresponding authors.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- DR:
-
Diabetic retinopathy
- LC–MS/MS:
-
Liquid chromatography–mass spectrometry
- PLS-DA:
-
Partial least squares discriminant analysis
- VIP:
-
Variable importance in the projection
- PSM:
-
Propensity score matching
- Cer:
-
Ceramides
- SM:
-
Sphingomyelins
- PC:
-
Phosphatidylcholine
- LPC:
-
Lysophosphatidylcholines
- MRM:
-
Multiple reaction monitoring
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- CHOL:
-
Total cholesterol
- TG:
-
Triglyceride
- HDL-c:
-
High density lipoprotein-cholesterol
- LDL-c:
-
Low density lipoprotein-cholesterol
- UA:
-
Uric acid
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- ALP:
-
Alkaline phosphatase
- GGT:
-
Gamma-glutamyl transpeptidase
- TBIL:
-
Total bilirubin
- DBIL:
-
Direct bilirubin
- TP:
-
Total protein
- ALB:
-
Albumin
- GLU:
-
Glucose
- BUN:
-
Blood urea nitrogen
- CRE:
-
Creatinine
References
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14.
Wong TY, Cheung CM, Larsen M, Sharma S, Simo R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012.
American Diabetes A. 10. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S105-S18.
Jampol LM, Glassman AR, Sun J. Evaluation and care of patients with Diabetic Retinopathy. N Engl J Med. 2020;382:1629–37.
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Beulens JW, Patel A, Vingerling JR, Cruickshank JK, Hughes AD, Stanton A, et al. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52:2027–36.
Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Reviews Endocrinol. 2017;13:79–91.
Zeng W, Beyene HB, Kuokkanen M, Miao G, Magliano DJ, Umans JG, et al. Lipidomic profiling in the strong heart study identified American indians at risk of chronic kidney disease. Kidney Int. 2022;102:1154–66.
Knuplez E, Marsche G. An updated review of Pro- and Anti-inflammatory properties of plasma lysophosphatidylcholines in the Vascular System. Int J Mol Sci. 2020;21.
Tonks KT, Coster ACF, Christopher MJ, Chaudhuri R, Xu AM, Gagnon-Bartsch J, et al. Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obesity. 2016;24:908–16.
Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 2018;19:175–91.
Chaurasia B, Tippetts TS, Mayoral Monibas R, Liu J, Li Y, Wang L, et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Volume 365. New York, NY: Science; 2019. pp. 386–92.
Klein RL, Hammad SM, Baker NL, Hunt KJ, Al Gadban MM, Cleary PA, et al. Decreased plasma levels of select very long chain ceramide species are associated with the development of nephropathy in type 1 diabetes. Metab Clin Exp. 2014;63:1287–95.
Fox TE, Han X, Kelly S, Merrill AH 2nd, Martin RE, Anderson RE, et al. Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy. Diabetes. 2006;55:3573–80.
Coen PM, Goodpaster BH. Role of intramyocelluar lipids in human health. Trends Endocrin Met. 2012;23:391–8.
Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286:27855–62.
Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M et al. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:2614–8.
Hyotylainen T, Oresic M. Analytical Lipidomics in metabolic and clinical research. Trends Endocrin Met. 2015;26:671–3.
Xuan Q, Ouyang Y, Wang Y, Wu L, Li H, Luo Y, et al. Multiplatform Metabolomics reveals novel serum metabolite biomarkers in Diabetic retinopathy subjects. Adv Sci (Weinh). 2020;7:2001714.
Hyotylainen T, Oresic M. Optimizing the lipidomics workflow for clinical studies-practical considerations. Anal Bioanal Chem. 2015;407:4973–93.
Grochowski ET, Pietrowska K, Godlewski A, Gosk W, Buczynska A, Wojnar M et al. Simultaneous comparison of aqueous humor and serum metabolic profiles of Diabetic and nondiabetic patients undergoing cataract Surgery—A targeted and quantitative Metabolomics Study. Int J Mol Sci. 2023;24.
Liew G, Lei Z, Tan G, Joachim N, Ho IV, Wong TY, et al. Metabolomics of Diabetic Retinopathy. Curr Diab Rep. 2017;17:102.
Sarafian MH, Gaudin M, Lewis MR, Martin FP, Holmes E, Nicholson JK, et al. Objective set of criteria for optimization of sample preparation procedures for ultra-high throughput untargeted blood plasma lipid profiling by ultra performance liquid chromatography-mass spectrometry. Anal Chem. 2014;86:5766–74.
Li J, Li L, Liu R, Zhu L, Zhou B, Xiao Y, et al. Integrative lipidomic features identify plasma lipid signatures in chronic urticaria. Front Immunol. 2022;13:933312.
Wen B, Mei Z, Zeng C, Liu S. metaX: a flexible and comprehensive software for processing metabolomics data. BMC Bioinformatics. 2017;18:183.
Fort PE, Rajendiran TM, Soni T, Byun J, Shan Y, Looker HC et al. Diminished retinal complex lipid synthesis and impaired fatty acid β-oxidation associated with human diabetic retinopathy. Jci Insight. 2021;6.
Fox TE, Han XL, Kelly S, Merrill AH, Martin RE, Anderson RE, et al. Diabetes alters sphingolipid metabolism in the retina - A potential mechanism of cell death in diabetic retinopathy. Diabetes. 2006;55:3573–80.
Levitsky Y, Hammer SS, Fisher KP, Huang C, Gentles TL, Pegouske DJ et al. Mitochondrial Ceramide effects on the retinal pigment epithelium in diabetes. Int J Mol Sci. 2020;21.
Mondal K, Mandal N. Role of bioactive sphingolipids in inflammation and Eye diseases. Adv Exp Med Biol. 2019;1161:149–67.
Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016;37:1967–76.
Summers SA, Chaurasia B, Holland WL. Metabolic messengers: ceramides. Nat Metabolism. 2019;1:1051–8.
Zhang QJ, Holland WL, Wilson L, Tanner JM, Kearns D, Cahoon JM, et al. Ceramide mediates vascular dysfunction in Diet-Induced obesity by PP2A-Mediated dephosphorylation of the eNOS-Akt complex. Diabetes. 2012;61:1848–59.
Wilmott LA, Grambergs RC, Allegood JC, Lyons TJ, Mandal N. Analysis of sphingolipid composition in human vitreous from control and diabetic individuals. J Diabetes Complications. 2019;33:195–201.
Shi AH, Yoshinari M, Wakisaka M, Iwase M, Fujishima M. Lysophosphatidylcholine molecular species in low density lipoprotein of type 2 diabetes. Horm Metab Res. 1999;31:283–6.
Canning P, Kenny BA, Prise V, Glenn J, Sarker MH, Hudson N, et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2) as a therapeutic target to prevent retinal vasopermeability during diabetes. Proc Natl Acad Sci U S A. 2016;113:7213–8.
Zhong J, Cheung CYY, Su X, Lee CH, Ru Y, Fong CHY, et al. Specific triacylglycerol, diacylglycerol, and lyso-phosphatidylcholine species for the prediction of type 2 diabetes: a ∼ 16-year prospective study in Chinese. Cardiovasc Diabetol. 2022;21:234.
Krischer JP, Liu X, Lernmark A, Hagopian WA, Rewers MJ, She JX, et al. The influence of type 1 diabetes genetic susceptibility regions, Age, Sex, and Family History on the Progression from multiple autoantibodies to type 1 diabetes: a TEDDY Study Report. Diabetes. 2017;66:3122–9.
Acknowledgements
Y.N.W. is supported by the China “Thousand Talents Plan” (Young Talents), Shaanxi province “Thousand Talents Plan” (Young Talents) and Foundation of Xi’an Jiaotong University (Plan A).
Funding
This study was supported by grants from The Natural Science Foundation Program of Shaanxi (2024JC-YBQN-0828) and National Key R&D Program of China (No. 2018YFC1311501).
Author information
Authors and Affiliations
Contributions
B.S., Y.W., and L.L. conceived this review and critically revised the manuscript. M.H., G.H., and M.L. drafted the manuscript. Z.P., H.G., Y.W., and J.S. drew the figures and collected the related references. H.L., X.Y., M.Z., Z.C. and P.C.N. supervised and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted with approval from the Institutional Review Board at the First Affiliated Hospital of Xi’an Jiaotong University, Shaanxi, China (approval number: XJTU1AF2018LSK-055). Written informed consent was obtained from all participants.
Consent for publication
All authors critically reviewed and approved the final manuscript.
Competing interests
All authors declared no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, M., Hou, G., Liu, M. et al. Lipidomic studies revealing serological markers associated with the occurrence of retinopathy in type 2 diabetes. J Transl Med 22, 448 (2024). https://doi.org/10.1186/s12967-024-05274-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05274-9