Abstract
Background
In this study, the prevalence of vitamin D deficiency was assessed in a group of children and adolescent patients with recent-onset type 1 diabetes mellitus (T1DM).
Methods
Fifty-three patients with age 8–18 years and duration of T1DM less than 8 weeks were recruited. A food frequency questionnaire (FFQ) was used to assess dietary vitamin D and calcium intake. Sunshine exposure was measured using a questionnaire to quantify the amount of time children spent in the sun and other sun-related habits, and a sun index score was generated. Serum 25(OH)D < 20 ng/ml was considered as vitamin D deficiency. Logistic regression was used to assess predictors of vitamin D deficiency.
Results
All patients were vitamin D deficient (77%) or insufficient (23%). In a logistic regression model, it was shown that the risk of being vitamin D deficient was significantly decreased by sunlight exposure ≥ 15 minutes during the weekends versus < 15 minutes (OR: 0.06, 95% CI: 0.01–0.75; P=0.029). In addition, vitamin D deficiency in boys was lower than girls in this model (OR: 0.164 [95% CI: 0.02–1.11]; P = 0.063).
Conclusion
Vitamin D deficiency is highly prevalent among children and adolescents with T1DM in Iran. Boys and children with ≥ 15 minutes sunlight exposure in weekends were less likely to be vitamin D deficient than girls and those with < 15 minutes sunlight exposure.
Similar content being viewed by others
Background
Type 1 diabetes mellitus (T1DM) is a complex disease characterized by autoimmune and progressive destruction of insulin secreting pancreatic β-cells. Both genetic and environmental factors are main agents participating in this autoimmune process [1, 2].
The incidence is very different according to geographic variation, with a child in Finland being about 400 times more likely than a child in Venezuela to acquire the disease [3]. Season is another factor, with lower incidence rate in the summer and statistically significant peaks in the late winter and early spring months [4].
One of the environmental factors thought to be protective against the development of T1DM is early supplementation with vitamin D [5]. Vitamin D, a potent regulator of calcium and phosphate metabolism, has been shown to possess immunomodulatory properties [6]. Several studies suggest that vitamin D supplementation in early childhood decreases the risk of developing T1DM [5, 6]. Moreover, there is a negative correlation between vitamin D intake of pregnant mother and the presence of islet antibodies in her child [7]. This observation suggests an immunological mechanism behind the association between vitamin D and T1DM.
The marker of vitamin D status is 25(OH)D since it is the major circulating metabolite of vitamin D [8]. Studies from different countries have shown a highly variable prevalence of vitamin D deficiency ranging from 15 to 60% among children and adolescents with T1DM [9–13]. Definition of vitamin D deficiency is controversial, however, most experts agree that 25(OH)D of <20 ng/ml is considered to be vitamin D deficiency, whereas a 25(OH)D of 21–29 ng/ml is considered to be insufficient, and a level ≥30 ng/ml is the normal value of vitamin D in both children and adults [14].
Before conducting a clinical trial of supplementing patients with T1DM, it is required to assess the existing status. In this cross-sectional study, the prevalence of vitamin D deficiency was assessed in children and adolescents with new-onset T1DM. Factors such as BMI, sex, sunlight exposure, and dietary vitamin D and calcium were assessed in relation to vitamin D deficiency. In addition, serum iPTH (intact parathyroid hormone) level was assessed to identify patients with secondary hyperparathyroidism.
Methods
Study design and patients
The research was approved by the Ethics Committee of Endocrinology and Metabolism Research Center (EMRC)/Tehran University of Medical Sciences. Fifty three patients with newly diagnosed T1DM were recruited from outpatient diabetes clinic in Shariati Hospital and Children Medical Center of Tehran University of Medical Sciences.
Subjects included if they were 8–18 year, consecutively diagnosed as having T1DM [15], duration of clinical disease less than 8 weeks, and without any medical co-morbidities or any other major chronic disease. Subjects were excluded from the study if they had consumed cholecalciferol, calcium, multi-vitamin or mineral supplementation, or vitamin D-fortified foods during the previous 3 months.
A 49-item semi-quantitative food frequency questionnaire (FFQ) on calcium and vitamin D intake was administered by the nutritionist. Frequencies of food intakes over the previous 3 months were reported in day, week, or month. Calcium content of foods was derived from Nutritionist III software modified for Iranian foods. Vitamin D was calculated according to the “Provisional Table on the Vitamin D Content of Foods” released by United States Department of Agriculture (USDA) [16].
Average sunshine exposure was measured using a questionnaire to quantify the amount of time children spent in the sun and other sun-related habits during past 3 months since patients’ recruitment. The questions asked about time spent outdoors, time of day when outdoors, use of sunscreen, sun protection factor (SPF), type of clothing, and the skin pigment. It was a modified version of the sun exposure questionnaire designed by Dr Glanz [17], and the questionnaire we used before [18].
Sun index score was generated as a measure combining hours spent in sun exposure during weekdays and weekends, and sun protective behaviors (i.e. clothing and sunscreen use) and patients’ skin color. Possible scores ranged from 4–14. Higher scores represented higher sunlight exposure. The sun scores were examined with respect to their relationship to serum vitamin D levels. Fasting serum 25(OH)D and intact PTH level was measured using immunoradiometric assay with the IDS kit (Boldon, Tyne and Wear, UK).
Statistical analysis
Data was analyzed using the statistical software SPSS version 16.0 for windows (SPSS Inc., Chicago, IL). P-values < 0.05 were considered statistically significant. Logistic regression was used to assess predictors of vitamin D deficiency. Serum 25(OH)D as a dichotomous variable (1 = Vitamin D deficient, and 0 = Vitamin D insufficient) was used to examine the associations of vitamin D deficiency with sex (0 = female, 1 = male), time duration in sun exposure in weekdays (0 = < 30 minutes, 1 = ≥ 30 minutes), time duration in sun exposure during weekend (0 = <15 minutes, 1 = ≥15 minutes), color of skin (0 = brown and olive, 1 = fair), and type of clothing (0 = sun exposure limited to hand and face, 1 = sun exposure more than hand and face).
Results
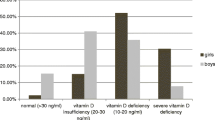
Patients were recruited at the end of summer and during autumn of Iran (September to December). The mean age of the patients was 10.3 ± 2.0 (8–14 years) and the mean duration since diagnosis of T1DM was 43 ± 15 days. Seventy-four percent of subjects were girls and 26% were boys.
Mean daily dietary calcium and vitamin D intake among patients was 931 ± 506 mg/day (range 197–2164 mg/day) and 38 ± 17 IU/day (range 13–76 IU). Daily vitamin D intakes in all patients were less than the recommended 200 IU per day for 9–13 year old children (Institute of Medicine, 1997). Calcium intake was not different between boys and girls (P = 0.860). Vitamin D intake was higher in boys than girls, but the difference was only borderline significant (P = 0.076).
Mean baseline sun index score among patients was 9.8 ± 1.5. The sun index score was significantly higher in boys than girls (11.0 ± 1.15 and 9.4 ± 1.5 respectively; P < 0.001). Figure 1 shows the scores of different components of sun index among girls and boys at baseline. The boys had significantly higher scores than girls for “hours outside exposed to sunlight” (3.08 ± 0.5 vs. 2.22 ± 0.28, P = 0.004), “use of sunscreen” (4.0 ± 0.0 vs. 3.55 ± 0.26, P = 0.001), and “type of clothing” (1.87 ± 0.18 vs. 1.33 ± 0.14, P < 0.001). In fact, the boys spent more time outside in the sun. In addition, their clothing was less covered than girls, and they never used sunscreen (all boys obtained score 4 for this item). The score of skin color did not differ between the boys and girls (P > 0.05).
Mean serum levels of 25(OH)D was 13.2 ± 6.1 ng/ml (range 4.1–27.4 ng/ml). According to these baseline values, 77% of patients were vitamin D deficient and the remaining 23% were vitamin D insufficient. No patient had sufficient level of serum 25(OH)D. There was a negative correlation between 25(OH)D concentration and iPTH level (r = −0.404, P = 0.003).
Serum vitamin D levels were significantly higher in boys compared to the girls (18.3 ± 7.8 and 10.8 ± 4.3 ng/ml, respectively; P = 0.004). In addition, there was a negative significant correlation between serum 25(OH)D and BMI (r = −0.291, P = 0.034). However, the difference of BMI values between vitamin D deficient and vitamin D insufficient subgroups did not reach a significant level (P = 0.132). Serum levels of 25(OH)D were not correlated with the age of the patients (r = − 0.148, P = 0.284).
Furthermore, serum 25(OH)D levels were not correlated with dietary vitamin D intake (r = 0.22, P = 0.349) and calcium intake (r = −0.27, P = 0.091). Likewise, there was no significant difference in dietary vitamin D intake among vitamin D deficient and vitamin D insufficient patients (P = 0.294). Calcium intake was higher in vitamin D deficient patients compared to vitamin D insufficient ones, but the difference was not significant (987 ± 531 and 729 ± 355 mg/day; P = 0.157).
There was a positive significant correlation between sun scores and serum levels of vitamin D (r = 0.37, P = 0.008). The sunlight index score was significantly lower in subjects with vitamin D deficiency compared to those with vitamin D ≥ 20 ng/ml (10.7 ± 1.4 and 9.5 ± 1.5, P = 0.026).
In a logistic regression model, the impact of sex and sun exposure behaviors was assessed on the likelihood of vitamin D deficiency. As shown in the Table 1, only time duration exposed to sunlight during the weekend had a statistically significant contribution to the model. In fact, the risk of being vitamin D deficient was significantly decreased by sunlight exposure ≥ 15 minutes during the weekends versus < 15 minutes (odds ratio [OR]: 0.06 [95% confidence interval [CI]: 0.01–0.75]; P = 0.03). The effect of sex on vitamin D status in this model was borderline significant, meaning that after adjustment for sun exposure-related behaviors, boys were less likely to be vitamin D deficient than girls (OR: 0.164 [95% CI: 0.02-1.11]; 0.10 > P > 0.05). The likelihood of being vitamin D deficient was not associated with the duration of sun exposure during weekdays, skin color, and type of clothing.
Discussion
An important finding of this study was the high prevalence of vitamin D deficiency (77%) compared to those in an Australian, US, Swiss, and Italian study [9–13], demonstrating 15–60.5% deficiency using the same cut-off values to define serum vitamin D status. This range of different prevalence might be explained by differences in geographical location, the age of patients, duration since diagnosis of diabetes, and glycaemic control as suggested recently [10].
Vitamin D deficiency is a common problem even in healthy children and adolescents at a variety of different latitudes [19–23]. Few studies have assessed vitamin D status of healthy children and adolescents in some cities of Iran, demonstrating 5% vitamin D deficiency among 513 healthy 6–to 7-year-old children in Isfahan [24], and serum 25-OHD < 20 ng/ml in 78% of healthy children and adolescents (8–18 years) from Tehran [25]. Similar study in 9–12-year-old primary-school children in Tehran found that 91.7% of children had 25(OH)D < 20 ng/ml during autumn and winter [26]. The age group in the latter study was very similar to our patients’. In addition, serum samples in that study obtained during autumn and winter, and mean serum 25(OH)D was 9.5 ± 8.9 ng/ml [26]. Likewise, our baseline serum samples were drawn at the end of summer and during the autumn, and serum vitamin D levels were comparable to the aforementioned survey (13.2 ± 6.1 ng/ml). In a study in Switzerland, vitamin D deficiency rose from 60.5% to 84.1% in winter in T1DM children [11], which shows the season as an important contributor to vitamin D status. Findings from studies in Iran imply that vitamin D deficiency is a highly prevalent problem among Iranian children and adolescents, whether they are healthy or with T1DM. In accordance with previous studies [26, 27], a negative correlation was observed between baseline 25(OH)D and iPTH concentrations.
Significant difference in 25(OH)D concentration between girls and boys in the present study was in agreement with previous research [25, 26]. This finding could be explained by difference in sun exposure between the two genders, as a higher sun index score was shown in boys. In addition, faster growth spurt during puberty in girls and more vitamin D requirement for bones might be a further reason for the higher prevalence of vitamin D deficiency among girls than boys [25].
A significant negative correlation was found between 25(OH)D levels and BMI as previously reported [26, 28, 29]. Higher BMI in children and adolescents is accompanied by rapid growth of bones as they are taller than average and their bone age is slightly advanced [29]. These children need more vitamin D because of the high expenditure that would result in low blood levels of this vitamin.
Vitamin D intake in this study included only intakes from foods, since we did not enroll patients with a history of vitamin D supplementation during the previous 3 months. In addition, food sources of vitamin D are very limited, unless they are fortified. There are some vitamin D fortified foods in Iran; however they are rare and expensive. Children participating in this study did not consume any kind of foods fortified with vitamin D. As a result, low intakes of vitamin D were expected. All participants reported a daily intake of vitamin D less than the recent RDA (600 IU/day) and even lower than previous DRI (200 IU/day) [30]. Similarly, surveys in 6–7 year-old children in Isfahan [24], and 11–15 year-old girls in Tehran [31] revealed suboptimal vitamin D intake, all of them suggesting urgent need for food fortification or vitamin D supplementation in the population of Iranian children and adolescents.
Dietary vitamin D intake can be an important contributor to vitamin D status, but the current study did not find any association between vitamin D intake and serum levels of 25(OH)D. There are similar findings which show only poor correlation between vitamin D intake and serum vitamin D [32, 33]. In contrast, dietary intake of vitamin D had significant effects on serum vitamin D in children in Isfahan [24]. These differences could be partly explained by differences in questionnaires or food composition tables used to estimate dietary intake, and the difference in age groups.
Low intake of calcium induces high serum PTH level and increases catabolism of 25(OH)D, therefore low intake of calcium causes decreased 25(OH)D [34]. The average daily dietary intake of calcium in this study (931 ± 506 mg/day) was almost near recommended values of 1300 mg for the 9- to 18-year- old age group. There was no correlation between calcium intake and serum 25(OH)D levels in the current trial.
Exposure to sunlight or UVB (Ultraviolet B) radiation is the primary source of vitamin D in the body [35]. In this study, both sun exposure and sun protective behaviors were included in the questionnaire to obtain a sun index score. A significant correlation between sun score and serum vitamin D levels was found. Similar results were obtained in previous studies demonstrating significant effects of sun exposure duration on serum levels of vitamin D [24]. Likewise, in a case–control study in multiple sclerosis patients in Australia [36] and a study in peri-menopausal women in Denmark [37], significant correlations was observed between various measures of self-reported sun exposure and serum 25(OH)D levels. However, correlation coefficients were low, suggesting that most of the variation in serum 25(OH)D concentrations was not explained by self-reported sun exposure. In contrast, some studies did not find significant relations between serum 25(OH)D and sunlight exposure as assessed by questionnaires [38, 39]. Different results can be partly explained by different sun questionnaires, different age groups, and different regions of study.
It was found that participants with more time exposed to sun during weekend had higher levels of serum vitamin D, suggesting that as a predictor of vitamin D status in this study. Several studies have also correlated outdoor activity with vitamin D status [40–42]. UVB radiation increases vitamin D levels, as it was described earlier. However, the duration of sun exposure during weekdays was not a significant predictor of serum vitamin D in the full model of regression. It may be explained by different type of clothing during weekdays and weekends, since wearing long sleeve shirt or dress is mandatory for children in the school, but not during weekends. Effects of skin color and type of clothing on serum 25(OH)D status was not significant after controlling for sex, and other variables in the model.
Conclusion
In summary, we observed a high prevalence of vitamin D deficiency or insufficiency among children and adolescents with T1DM. However, lack of a comparison group is the main limitation of this study, since it has been shown that vitamin D deficiency is highly prevalent in healthy children in Iran. Time duration of sunlight exposure during weekends and sex were the main predictors of serum vitamin D deficiency. Boys and children with ≥ 15 minutes exposed to sunlight in weekends were less likely to be vitamin D deficient than girls and those with < 15 minutes exposing to sunlight. Dietary vitamin D and calcium intake did not have any effect on serum vitamin D.
References
Pugliese A: Genetics of type 1 diabetes. Endocrinol Metab Clin North Am 2004, 33(1):1–16. https://doi.org/10.1016/S0889-8529(03)00082-3
Park Y: Prediction of the risk of type 1 diabetes from polymorphisms in candidate genes. Diabet Res Clin Pract 2004, 66: 19–25.
Gillespie KM: Type 1 diabetes: pathogenesis and prevention. CMAJ 2006, 175: 165–170. https://doi.org/10.1503/cmaj.060244
Karvonen M, Tuomilehto J, Libman I, R LaPorte for WHO DiaMond Project Group: A review of the recent epidemiological data on incidence of type 1 (insulin-dependent) diabetes mellitus worldwide. Diabetologia 1993, 36: 883–892. https://doi.org/10.1007/BF02374468
Mathieu C, Gysemans C, Giulietti A: Vitamin D and diabetes. Diabetologia 2005, 48(7):1247–1257. https://doi.org/10.1007/s00125-005-1802-7
Deluca HF, Cantorna MT: Vitamin D: its role and uses in immunology. FASEB J 2001, 15(14):2579–2585. https://doi.org/10.1096/fj.01-0433rev
Fronczak CM, Barón AE, Chase HP, et al.: In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care 2003, 26(12):3237–3242. https://doi.org/10.2337/diacare.26.12.3237
Holick MF: Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 2004, 79: 362–371.
Greer RM, Rogers MA, Bowling FG, et al.: Australian children and adolescents with type 1 diabetes have low vitamin D levels. Med J Aust 2007, 187(1):59–60.
Svoren BM, Volkening LK, Wood JR, et al.: Significant vitamin D deficiency in youth with type 1 diabetes mellitus. J Pediatr 2009, 154(1):132–134. https://doi.org/10.1016/j.jpeds.2008.07.015
Janner M, Ballinari P, Mullis PE, et al.: High prevalence of vitamin D deficiency in children and adolescents with type 1 diabetes. Swiss Med Wkly 2010, 140: w13091.
Pozzilli P, Manfrini S, Crinò A, IMDIAB group, et al.: Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm Metab Res 2005, 37(11):680–683. https://doi.org/10.1055/s-2005-870578
Bizzarri C, Pitocco D, Napoli N, IMDIAB Group, et al.: No protective effect of calcitriol on beta-cell function in recent-onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care 2010, 33(9):1962–1963. https://doi.org/10.2337/dc10-0814
Holick MF: Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 2009, 19(2):73–78. https://doi.org/10.1016/j.annepidem.2007.12.001
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003, 26(Suppl 1):5–20.
United States Department of Agriculture (USDA) Nutrient Data Laboratory: Krause’s food & nutrition therapy. In Provisional table on the vitamin D content of foods. 10th edition. Edited by: Mahan LK, Escott-Stump S. Philadelphia: Saunders; 2000:1144–1145.
Glanz K, Yaroch AL, Dancel M, et al.: Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol 2008, 144(2):217–222. https://doi.org/10.1001/archdermatol.2007.46
Ataie-Jafari A, Hossein-nezhad A, Maghbooli Z, et al.: The influence of sunlight exposure on serum vitamin D concentration and bone turnover; a controlled clinical trial. Iranian J Health 2008, 37: 41–48.
El-Hajj Fuleihan G, Nabulsi M, Choucair M, et al.: Hypovitaminosis D in healthy schoolchildren. Pediatrics 2001, 107(4):E53. https://doi.org/10.1542/peds.107.4.e53
Du X, Greenfield H, Fraser DR, et al.: Vitamin D deficiency and associated factors in adolescent girls in Beijing. Am J Clin Nutr 2001, 74: 494–500.
Gordon CM, DePeter KC, Feldman HA, et al.: Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 2004, 158: 531–537. https://doi.org/10.1001/archpedi.158.6.531
Weng FL, Shults J, Leonard MB, et al.: Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr 2007, 86(1):150–158.
Ward LM, Gaboury I, Ladhani M, et al.: Vitamin D-deficiency rickets among children in Canada. CMAJ 2007, 177(2):161–166. https://doi.org/10.1503/cmaj.061377
Ardestani PM, Salek M, Keshteli AH, et al.: Vitamin D status of 6–to 7-year-old children living in Isfahan, Iran. Endokrynol Pol 2010, 61(4):377–382.
Razzaghy-Azar M, Shakiba M: Assessment of vitamin D status in healthy children and adolescents living in Tehran and its relation to iPTH, gender, weight and height. Ann Hum Biol 2010, 37(5):692–701. https://doi.org/10.3109/03014460903527348
Neyestani TR, Hajifaraji M, Omidvar N, et al.: High prevalence of vitamin D deficiency in school-age children in Tehran: a red alert. Public Health Nutr 2011, 28: 1–7. https://doi.org/10.1111/j.1525-1446.2010.00934.x
Cheng S, Tylavsky F, Kroger H, et al.: Association of low 25-hydroxy vitamin D concentrations with elevated parathyroid hormone concentration and low cortical bone density in early pubertal and pre-pubertal Finnish girls. Am J Clin Nutr 2003, 78: 485–492.
Bischof MG, Heinze G, Vierhapper H: Vitamin D status and its relation to age and body mass index. Horm Res 2006, 66(5):211–215. https://doi.org/10.1159/000094932
Hintz RL: Clinical pediatric endocrinology. In Management of disorders of size. 4th edition. Edited by: Brook CGD, Hindmarsh PC. London: Blackwell Science; 2001:124–139.
Institute of Medicine. Dietary Reference Intakes for Vitamin D and Calcium: Washington. DC: National Academies Press; 2011.
Dahifar H, Faraji A, Ghorbani A, et al.: Impact of dietary and lifestyle on vitamin D in healthy student girls aged 11–15 years. J Med Invest 2006, 53(3–4):204–208.
Takeuchi A, Okano T, Ishida Y, et al.: Effects of dietary vitamin D intake on plasma levels of parathyroid hormone and vitamin D metabolites in healthy Japanese. Miner Electrolyte Metab 1995, 21(1–3):217–222.
Thomas MK, Lloyd-Jones DM, Thadhani RI, et al.: Hypovitaminosis D in medical inpatients. N Engl J Med 1998, 338(12):777–783. https://doi.org/10.1056/NEJM199803193381201
Lips P: Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr Rev 2001, 22: 477–501. https://doi.org/10.1210/er.22.4.477
Grant WB, Holick MF: Benefits and requirements of vitamin D for optimal health: a review. Alt Med Rev 2005, 10: 94–111.
Van der Maei IAF, Blizzard L, Ponsonby A-L, et al.: Validity and reliability of adult recall of past sun exposure in a case–control study of multiple sclerosis. Cancer Epidemiol Biomarkers Prev 2006, 15(8):1538–1544. https://doi.org/10.1158/1055-9965.EPI-05-0969
Brot C, Vestergaard P, Kolthoff N, et al.: Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr 2001, 86: S97-S103. https://doi.org/10.1079/BJN2001345
Hashemipour S, Larijani B, Adibi H, et al.: Vitamin D deficiency and causative factors in the population of Tehran. BMC Publ Health 2004, 25(4):38.
Brady HL: Vitamin D and the vitamin D receptor gene in children at increased risk of developing type 1 iabetes. USA: PhD thesis, University of Colorado Health Sciences Center; 2006.
Scragg R, Camargo CA: Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2008, 168: 577–586. https://doi.org/10.1093/aje/kwn163
Jacobs ET, Alberts DS, Foote JA, et al.: Vitamin D insufficiency in southern Arizona. Am J Clin Nutr 2008, 87: 608–613.
Sahota H, Barnett H, Lesosky M, et al.: Association of vitamin D related information from a telephone interview with 25-hydroxyvitamin D. Canc Epidemiol Biomarkers Prev 2008, 17(1):232–238. https://doi.org/10.1158/1055-9965.EPI-07-0632
Acknowledgement
This study was supported by a grant from Endocrinology and Metabolism Research Center, Tehran University of Medical Sciences (TUMS). We thank patients participated in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AA-J, design, data analysis, and interpretation, AR, design, FA, design, SCL, interpretation, MQ, interpretation, BL, design and revision. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ataie-Jafari, A., Rahmat, A.B., Abbasi, F. et al. Vitamin D status and associated factors in recent-onset type 1 diabetic children in Iran. J Diabetes Metab Disord 11, 12 (2012). https://doi.org/10.1186/2251-6581-11-12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6581-11-12