Abstract
Human immunodeficiency virus (HIV) is still a serious global health concern responsible for more than 25 million deaths in last three decades. More than 34 million people are living with HIV infection. Macrophages and CD4+ T cells are the principal targets of HIV-1. The pathogenesis of HIV-1 takes different routes in macrophages and CD4+ T cells. Macrophages are resistant to the cytopathic effect of HIV-1 and produce virus for longer periods of time. In addition, macrophages being present in every organ system thus can disseminate virus to the different anatomical sites leading to the formation of viral sanctuaries. Complete cure of HIV-1 needs better understanding of viral pathogenesis in these reservoirs and implementation of knowledge into robust therapeutic products. In this review we will focus on the unique relationship between HIV-1 and macrophages. Furthermore, we will describe how successful antiretroviral therapy (ART) is in suppressing HIV and novel molecular and cellular strategies against HIV-1 in macrophages.
Similar content being viewed by others
Introduction
Human immunodeficiency virus type 1 (HIV-1) can infect several types of immune cells, however macrophages and CD4+ T lymphocytes cells are the principal targets of HIV-1 in human body [1, 2]. Macrophages are terminally differentiated immune cells which play an important role in the clearing of pathogens and cellular debris by phagocytosis. Besides, they also act as the antigen presenting cells and present processed pathogen antigen peptides to the CD4+ T cells via MHC II pathway [3, 4]. This exchange of information between macrophages and CD4+ T cells also has important role in the transmission of HIV-1 from macrophage to CD4+ T cells [5–7]. In addition, HIV-infected macrophages release soluble cytotoxic factors that can promote the apoptosis of bystander cells for example CD4+ and CD8+ T cells [8, 9].
HIV-1 infection results in the lysis of T lymphocytes (CD4+ T and CD8+ T cells) leading to their depletion, a hallmark of HIV-1 pathogenesis. On the contrary, macrophages are relatively less prone to the cytopathic effect of the virus [10, 11]. Since the life span of HIV-1 infected macrophage is long, thus they act as a source of virus production for longer period of time in infected patients [12]. In addition, macrophages are virtually present in every organ system (although with different names), thus can disseminate HIV-1 throughout the body of infected persons including brain [13]. Therefore, how HIV-1 interacts with macrophages and governs its life cycle in macrophage environment is very important. In this review we will summarize the interplay of HIV-1 and macrophages and therapeutic interventions against HIV-1 in macrophages.
Review
HIV-1 replication in the macrophage
HIV-1 entry into macrophages
First step of HIV-1 entry into target host cells involves virus ligand (virus surface glycoprotein gp120) and its interaction with CD4 receptor which is present in both T cells as well as in macrophages [14, 15] (Figure 1). Second step involves the fusion of viral envelope with host cell membrane which is governed by the engagement of the co-receptors (CCR5 or CXCR4) (Figure 1). Earlier it was believed that macrophages have CCR5 receptor and most of the T cells have CXCR4 receptor resulted in macrophage tropic and T cell tropic HIV-1 terminology [1]. Further studies revealed that both the co-receptors are present on macrophages as well as in T cells in vivo[1, 11, 16, 17]. Notably the naturally transmitted HIV-1 viruses utilize CCR5 for their infection, even though their primary targets are T cells not macrophages. In CNS, microglia (resident macrophages of the brain) are infected via CCR5 co-receptor. Common consensus is that these R5 and X4 viruses can replicate in both macrophages as well as in T cells. However, their replication efficiency varies in cell types which depend upon the cellular environment. Furthermore, viral progeny from macrophages and T cells can be identical however, they may have different sets of host protein incorporated in their viral particle (reviewed comprehensively in [1]).
Depicting key events of HIV-1 life cycle targeted by anti-retroviral drugs. The anti-retroviral drugs target four critical steps of the viral life cycle which are fusion (or entry) of virion in the susceptible cell, reverse transcription, integration of proviral DNA into host chromatin and polyprotein processing by viral encoded protease. Depending upon the steps they target, the anti-retroviral drugs are termed as fusion (entry) inhibitors (a), reverse transcriptase inhibitors (b), integrase inhibitors (c) and protease inhibitors (d). Targeting single step at a time usually results in the emergence of resistant mutants. ART is formulation of these inhibitors which suppresses HIV-1 growth to a significant extent. Please note that virus assembly in macrophages takes place at both plasma membrane (e) as well as in virus containing compartments (f)[47]. Only key proteins involved in HIV-1 life cycle in macrophage have been shown. Abbreviations: RT- reverse transcriptase, MA- matrix protein, IN- Integrase, Vpr-Viral protein R, P- virus encoded protease, PIC- Pre-integration complex, MVB- multi vesicular bodies, LE- late endosomes and VCC- virus containing compartment.
Reverse transcription and host restriction factors
Whether HIV-1 enters via CXCR4 or CCR5 coreceptor, in both cases the viral ribonucleoprotein complex is released into the cytoplasm [11, 18] where virus encoded reverse transcriptase using viral genomic RNA as template, generates single stranded cDNA followed by double stranded (ds)DNA [19, 20] (Figure 1). However, the rate of reverse transcription is slower in macrophages than what is observed in T cells. Macrophages being terminally differentiated non dividing cells have limited dNTP pools for proviral DNA production [21, 22]. Several reports have shown that addition of deoxynucleosides to the primary human macrophage culture remarkably enhances the rate of HIV-1 reverse transcription proving that dNTP pool is an important rate limiting factor in macrophages [21, 23, 24].
Additionally, macrophages possess certain inhibitory factors which interfere with viral life cycle and are termed as host restriction factors [25, 26]. These host restriction factors include tetherin, APOBEC3G and recently identified sterile alpha motif (SAM) domain and HD domain-containing protein 1 (SAMHD1) [25–27]. APOBEC3G is known to trigger G-to-A hypermutation in nascent DNA. Tetherin (also called CD317/BST-2) hinders the release of viral progeny from infected cells [26]. HIV-1 employs several strategies to overcome these restriction factors (reviewed in [28, 29]). HIV-1 accessory protein Vif and Vpu counteracts the APOBEC3G and tetherin respectively [4, 25, 26]. Even there are reports describing tetherin antagonism by HIV-1 Nef protein [30, 31].
SAMHD1 is a macrophage specific host restriction factor which has triphosphohydrolase activity resulting in hydrolysis of dNTPs into nucleosides and triphosphates. Thus SAMHD1 reduces the dNTPs pool in macrophages to a certain level resulting in the inefficient reverse transcription of HIV-1 genomic RNA into proviral DNA [32]. However, Vpx protein of HIV-2 induces proteasome-dependent degradation of SAMHD1 through CRL4DCAF1 E3 ubiquitin ligase [27]. Recently McKnight research group, in order to search for host restriction factors, screened several human genes and identified 114 genes with significant impact on HIV-1 replication. Furthermore, their studies revealed that inhibition of all members of PAF1 family resulted in increase in HIV-1 replication. Notably PAF1 is not restricted to macrophages only, they are also expressed in primary monocytes and T-lymphocytes, suggesting exhaustive list of restriction factors against HIV-1 [33]. Recently Allouch and colleagues showed that cyclin-dependent kinase inhibitor p21 inhibits HIV-1 replication in monocyte-derived macrophages (MDMs) by interfering with reverse transcription of the viral genome by a mechanism independent of SAMHD1. Additionally, they demonstrated that p21 curtails the dNTP synthesis through the down regulation of the expression of RNR2 (a subunit of ribonucleotide reductase) necessary for the biosynthesis of dNTPs [34].
Nuclear transport
Newly synthesized HIV dsDNA is imported to the nucleus as pre-integration complex (PIC) (Figure 1). Unlike T cells, in macrophages PIC transport to the nucleus is independent of cell division. PIC comprises of viral proteins which includes reverse transcriptase, Vpr, integrase (IN), matrix (MA, p17) and capsid protein (CA) in addition to newly synthesized dsDNA. However, CA dissociates from PIC prior to the nuclear entry. Vpr, IN and MA direct the transport PIC through nuclear pore mediated by importin α/β [35, 36] (Figure 1). However, precise function of these proteins in PIC nuclear transport is still a matter of debate [11]. Unlike IN and MA, Vpr lacks nuclear localization signal [37, 38]. In addition, interaction between importin α and Vpr is critical not only for the nuclear transport of PIC but also for the replication of HIV-1 in macrophages [39]. Furthermore, in primary macrophages, host cell protein emerin (an integral nuclear inner membrane protein) plays an indispensible role in integration of viral DNA into the chromatin [40, 41]. Primary macrophages lacking emerin have poor rate of HIV proviral DNA integration into the host chromatin however, lack of emerin does not inhibit PIC entry into the nucleus [40]. In addition, binding partners of emerin, the LEM (LAP2 (lamina-associated polypeptide 2)/emerin/MAN1) is necessary for the interaction of viral cDNA with emerin and capability of emerin to support HIV-1 infection in macrophages [40]. However, Shun and colleagues demonstrated that HIV-1 can efficiently infect dividing cells despite of the absence of emerin, suggesting the role of emerin in HIV-1 infection restricted to only macrophages [42]. Besides several other host factors are involved in the HIV life cycle in macrophages have been reviewed recently [43].
HIV-1 transcription
HIV-1 transcription is governed by binding of viral proteins and host factors to the long terminal repeat (LTR) of the virus, which functions as viral promoter [44]. Host factors include nuclear factor kappa B (NF-κB) family, AP-1 (activator protein 1), Sp family, C/EBP (CCAAT enhancer binding protein and NFAT (nuclear factor of activated T cells). These host factors have specific binding sites present on LTR. On the other hand, viral proteins Tat and Vpr also bind to the LTR to govern HIV-1 transcription [20, 44]. Worth mentioning, host factors could be cell type specific, for example C/EBP proteins and their binding sites are critical for HIV-1 replication in macrophages but not in CD4+ T cells [45]. In addition, primary macrophages infected with HIV-1 having mutation in C/EBP binding sites does not support HIV-1 replication. On the other hand, primary CD4+ T cells, Jurkat and H9 cells support the replication of HIV-1C/EBP mutants [45].
HIV-1 assembly in macrophages
In case of primary CD4+ T cells, HIV-1 assembly takes place at the plasma membrane [46]. On the other hand, the corresponding site in macrophages is not yet fully characterized [47]. Initial studies demonstrated the presence of HIV-1 virion particles in multivesicular bodies (MVBs) or late endosomes (LEs) like structures [47, 48] (Figure 1). Even immuno-electron microscopy studies supported latter finding as their studies revealed the presence of MVB specific markers (for example CD53, CD9, tetraspanins, CD81 and MHC II) in those structures [47, 49–51]. In addition, HIV-1 progeny released from infected macrophages also possess these markers, further strengthening the view that macrophages are released from LEs or MVBs [47, 50, 52]. However, several studies revealed that structures harboring HIV-1 in infected macrophages have some distinct characters which are not characteristics of LEs or MVBs. These unique characteristics include tubular connection to the extracellular space and neutral pH [53]. The term ‘virus containing compartments’ (VCCs) has been assigned to the structures which act as the site for the virus assembly in macrophages [47] (Figure 1). Interestingly, these VCCs are also present in uninfected macrophages however, they become more prominent upon HIV-1 infection [51, 53]. Worth mentioning, VCCs have limited access to the innate and adaptive immune effector molecules [47]. In contrast, several studies are in the favor of budding of HIV-1 progeny from plasma membrane in infected macrophages [54]. Taken together, these contrasting studies indicate that there is a fair possibility that HIV-1 may bud from plasma membrane as well as from VCCs (Figure 1). VCCs may act as a safe house for HIV-1 in macrophages leading to HIV-1 reservoirs. However, elegant experiments are further required to support this hypothesis.
Interplay between HIV proteins and cell signaling in macrophages
Among HIV-1 proteins, the viral proteins Tat, Vpr and Nef interfere with signaling pathways in macrophages.
Tat
The trans-activator of transcription (Tat) protein is a 86–101aa virus encoded pleiotropic protein which directly or indirectly modulates several steps of HIV life cycle including replication, transcription and progeny release by regulating both cellular as well as viral gene expression [20, 55–57]. In addition, Tat has been detected in sera of HIV infected patients as well in cell culture settings indicating its role as a modulator of cellular function in infected cells and also to target bystander cells [20, 58]. Furthermore, monocytes, macrophages and microglia are activated by Tat protein [20]. In addition, Tat is known to trigger the expression of HIV coreceptors (CXCR4, CCR5 and CCR3) in macrophages in a dose-dependent manner which might positively influence HIV-1 infection [59]. Furthermore, Tat acts as a potent chemoattractant for monocytes, macrophages and dendritic cells [60, 61]. Tat induces the production and release of tumor necrosis factor alpha (TNF-α) from macrophages [62]. Further, Tat mediated TNF-α induction was NF-kappa B (NF-κB) dependent and mediated through activation of signaling cascades including PLC (phospholipase C), protein kinase A and protein tyrosine kinase [20]. In addition, Tat enhances the endogenous levels of Ca2+ in macrophages which may subsequently lead to the production of chemokines and pro-inflammatory cytokines [63]. Latter events may be responsible for HIV-1 induced neuropathogenesis and inflammation [64].
Viral protein R (Vpr)
Vpr is a virion-associated protein dispensable for viral replication in T cells however is indispensible for viral replication in macrophages [65]. Vpr has been localized in cytoplasm as well as in nucleus of the infected cells [66]. Vpr is a multifunctional protein which regulates viral replication, cellular events like NF-κB-mediated transcription, apoptosis and cytokine production [20, 67]. Effect of recombinant Vpr (rVpr) has been demonstrated in macrophages. Although high concentration of rVpr resulted in significant cytotoxicity in macrophages however, at lower concentration rVpr has been shown to increase the biological activity of several transcription factors including NF-κB, c-Jun and AP-1 in promonocytic cells and primary macrophages [68]. In addition rVpr stimulates HIV-1 replication in acutely infected primary macrophages. Furthermore, infection of macrophages with Vpr-deficient viral mutants resulted in decreased production of p24 which can be corrected by addition of rVpr [69]. Moreover, Vpr independently enhances the expression of cyclin-dependent kinase inhibitor 1A (CDKN1A/p21) in macrophages whereas Vpr mutants exhibit lack of upregulation of p21 and display reduced viral replication [70]. Taken together, data strongly suggest that Vpr enhances the viral replication in acutely and latently infected macrophages.
Nef
Nef is expressed during early life cycle of HIV-1. Nef is a 27 kDa myristoylated protein required for efficient viral replication in infected cells [71, 72]. In addition, Nef enhances the survival of infected cells which helps in the expansion of infectious viral population. Furthermore, Nef hampers the immune system of infected patients by several mechanisms including down-regulating the expression of MHC I, MHC II, CD28, CD4 [73, 74] and by activating PI3K [75]. Nef down-regulates the expression of CD4 receptor in macrophages which serves two purposes. Firstly, CD4 down-regulation in infected cells may promote the release of viral progeny by avoiding sequestration of viral envelope by CD4 [76]. Secondly, it helps in avoiding superinfection which otherwise could lead to premature cell death [71, 76].
In monocyte derived macrophages (MDMs) exogenously added recombinant Nef (rNef) regulates the expression of several genes in a short time span (2 hours). These findings indicate a robust transcriptional programming governed by Nef protein leading to the production and secretion of soluble factors which in turn activates STAT1 and STAT3 in primary monocytes/macrophages [20, 77]. Similarly, addition of rNef to the MDMs cultures resulted in the rapid induction of transcription factors NF-κB, AP-1, and c-Jun N-terminal kinase and enhanced HIV-1 transcription. Furthermore, in vitro treatment of macrophages with rNef has been reported to trigger IKK/NF-κB, MAPK and IRF-3 signaling cascades. Additionally, Nef induces robust phosphorylation of MAPKs, including ERK1/2, JNK, and p38 [20, 78]. Notably, the role of Nef in HIV-HCV coinfected macrophages has been recently described [79].
Contribution of macrophages to HIV-1 pathogenesis
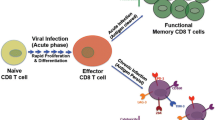
HIV-1 pathogenesis is characterized by progressive cell depletion involved in adaptive immunity including CD4+ T and CD8+ T cells [8, 9]. Not only HIV-infected CD4+ T cells are lysed but uninfected CD4+ T cells more prominently undergo apoptosis [80] (Figure 2). Nef plays dual role in HIV-1 pathogenesis. On one hand, Nef protects HIV-infected cells from cell death to favor efficient viral production. On the other hand, Nef induces apoptosis in bystander CD4+ T cells. Furthermore, it has been shown that Nef-expressing macrophages release paracrine factors including soluble ICAM and CD23 which increase the lymphocytes permissively for HIV-1 infection [81] (Figure 2). Additionally, Nef induces the expression of Fas ligand (CD95L) on the surface of infected T cells. Furthermore, interaction between CD95L and its receptor present on cells in close vicinity triggers apoptosis in bystander cells [8, 82] (Figure 2). Notably, Nef protects infected cells from apoptosis via CD95-CD95L cis interaction by inhibiting ASK1 (apoptosis signal-regulating kinase 1), caspase 8 and caspase 3 activation [20, 83] (Figure 2). Worth mentioning, ASK1 is a common partner of Fas and TNF-α mediated death signaling cascades [83].
Relationship between macrophages and T lymphocytes in HIV-1 infection. Macrophages harboring HIV-1 play an important role in HIV pathogenesis. Nef stimulates the release of soluble factors ICAM and CD23 which makes uninfected CD4+ T cells more susceptible to HIV infection, thereby favoring the expansion of the viral reservoir (a). In addition, Nef induces the expression of Fas ligand (FasL, CD95L) on HIV-infected cells. Interaction of CD95L and its receptor (Fas) present on uninfected CD4+ T cells results in apoptosis (b). On the other hand in infected CD4+ T cells, Nef inhibits the expression of proteins involved in apoptosis including ASK1, caspase 8 and caspase 3 (c), protects infected CD4+ T cells from cell death and further expands the viral reservoir. HIV regulatory protein Tat stimulates the production and release of TRAIL from the infected macrophages. TRAIL binds with its receptor (DR5) present on uninfected CD4+ T cells and induces apoptosis (d). Furthermore, gp120 interaction with CXCR4 receptor increases the expression of TNF-α on macrophages which interacts with TNFR2 present on CD8+ T cells. This interaction results in the down regulation of the anti-apoptotic protein Bcl-XL and ultimately leads to apoptosis (e). Moreover, HIV infection in macrophages is known to induce macrophage colony stimulating factor (M-CSF) which inhibits the expression of TRAILR1 on macrophages and upregulates the expression of anti-apoptotic proteins (f), favoring the resistance to apoptosis of infected macrophages. Therefore, targeting M-CSF has been suggested to increase apoptosis in infected macrophages.
In addition, uninfected macrophages have been shown to confer resistance against apoptosis in productively infected CD4+ T cells. Although expression of Nef by these infected CD4+ T cells is necessary for anti-apoptotic behavior however, presence of macrophages further enhances the number of non-apoptotic cells via intercellular contacts mediated by TNF stimulation [84]. This may be the one of the mechanisms of promotion of HIV-1 reservoir in T cells by macrophages. Another regulatory protein of HIV, Tat has been reported to stimulate the expression of TRAIL TNF related apoptosis-induced ligand (TRAIL) in U937, monocytes and primary macrophages [85, 86], which results in the apoptosis of uninfected cells (Figure 2). This finding provides an insight into another mechanism of elimination of bystander cells.
Recombinant glycoprotein gp120 (rgp120) (from X4 strain) has been reported to induce apoptosis of cytotoxic T cells (CTLs, CD8+ T cells). Furthermore, apoptosis is mediated by interaction between TNFR-2 present on the CD8+ T cells and TNF-α bound on the surface of macrophages [9] (Figure 2). In addition, the expression of TNFR-2 and TNF-α is positively regulated by treatment with rgp120 or upon HIV infection [9]. Moreover, stimulation of TNFR-2 receptor in primary T cells resulted in the down-regulation of anti-apoptotic protein Bcl-XL which may further explain CD8+ T cell elimination [87].
These results collectively revealed that macrophages play a central role in the propagation of HIV-1 infection, in depletion of CD4+ and CD8+ T cells, and in conferring anti-apoptotic characteristics to the HIV infected cells thereby favoring the expansion of the viral reservoir.
Macrophages and cytotoxic T cells (CTLs)
HIV-1 specific cytotoxic T cells (CTLs) play an important role in controlling HIV-1 infection during early stage of infection [88, 89]. CTLs act on the information provided by CD4+ T cells or antigen presenting cells [90]. However, in HIV-1 infected patients even effective CTLs response is also hampered. Studies showed that Nef downregulates the expression of HLA class I molecule in infected CD4+ T cells resulting in their escape from HIV-1 specific CTLs [91]. Interestingly, Fujiwara and Takiguchi, in their in vitro study demonstrated that HIV-1 specific CTLs are capable of effectively suppressing R5 virus replication in infected macrophages [92]. Furthermore, their data revealed that HIV-1 infected macrophages induce more proliferation of HIV-1 CTLs as compared to infected CD4+ T cells. Taken together data suggest the involvement of effective response of macrophages during early phase of HIV-1 infection [92]. However, in vivo the role of HIV-1 infected macrophages is largely influenced by their activation states [15]. Notably, macrophages are proposed to be in three kinds of activation states which are designated as M1 (pro-inflammatory in nature), M2 (anti-inflammatory in nature) and deactivated macrophages. Of note, M1 macrophages produce cytokines IL-23, IL-12, IL1-β, TNF-α and support Th1 response [15, 93, 94]. On the other hand, in M2 activation state, macrophages secrete IL-10 and support Th2 responses [15, 94]. According to proposed model, during early stage of HIV-1 infection, M1 activation is predominant which favors robust HIV-1 transcription and formation of viral reservoirs [15]. As the infection progressed, M1 state is off and M2 activation state is predominant followed by deactivation of macrophages resulting finally in failure in presenting antigen to the CTLs [15].
Search for apoptosis inducing agents in HIV-infected macrophages
Induction of apoptosis in chronically infected T cells has been suggested as a possible cure for HIV infection [95, 96]. Several new targets have been suggested in T cells, alteration of which can induce programmed cell death in infected T cells [97–99]. Vigorous efforts are also required to search for similar targets in infected macrophages.
HIV-1 infection in macrophages has been reported to induce the production of macrophage colony stimulating factor (M-CSF). Furthermore, M-CSF positively regulates the expression of anti-apoptotic proteins (Bfl-1 and Mcl-1) and inhibits the expression of death receptor TRAIL-R1 (Figure 2). Additionally, targeting of M-CSF has been also reported to enhance the apoptosis in macrophages [100]. In another recent report, apoptotic effect of viral protein Vpr has been examined in MDMs and THP1 macrophages. Their finding revealed that Vpr is not able to induce apoptosis in MDMs and THP1. Unlike undifferentiated cells, Vpr does not down regulate the expression of Bcl2 and inhibitors of apoptosis (IAPs) family members in macrophages [101]. Furthermore, down regulation of IAP1 and IAP2 make the macrophages susceptible for Vpr meditated apoptosis. Altering IAP activity has been suggested as a possible way to induce apoptosis in infected macrophages [101].
Conventional therapies against HIV-1 in macrophages
Currently, combinatorial antiretroviral therapy (ART) is widely used in suppressing HIV-1 infection to a significant level [102, 103]. ART has made a remarkable contribution in improving and enhancing life span of infected patients [104]. HIV-1 growth kinetics is different in macrophages and T cells suggesting varied impact of antiretroviral drugs against HIV-1 in these target cells. Here we will briefly describe the potential contribution of ART in HIV-infected macrophages.
Reverse transcriptase inhibitors (RTIs)
More than 25 compounds have been licensed for treating HIV in infected patients [105]. Out of them nearly fifty percent are reverse transcriptase inhibitors (RTIs) [105]. RTIs are of two types which are nucleoside reverse transcriptase inhibitors (NRTIs) and non nucleoside reverse transcriptase inhibitors (NNRTIs) [13].
Nucleoside reverse transcriptase inhibitors (NRTIs)
NRTIs target reverse transcriptase enzyme which is responsible for conversion of HIV genomic RNA into cDNA, an important step in the life cycle of HIV (Figure 1). NRTIs include emtricitabine, tenofovir, abacavir, lamivudine, stavudine, zalcitabine, didanosine and didovudine [105].
NRTIs mimic and compete with natural nucleotides pool for incorporation into growing chain of nascent HIV DNA. Notably, NRTIs require intracellular phosphorylation for conversion into functional inhibitors of HIV. Since most of NRTIs lacks 3′ OH moiety, therefore their incorporation into nascent HIV DNA leads to termination of DNA chain formation. Efficacy of these NRTIs majorly depends upon the levels of dNTPs pools [13, 24]. As discussed earlier, macrophages being terminally differentiated non dividing cells have limited pools of dNTPs as compared to actively dividing cells [13, 106]. Therefore, theoretically in this scenario, NRTIs will face less competition with natural dNTPs in macrophages. That may be the one of the reasons for better efficacy of NRTIs in macrophages as compared to CD4+ T cells [21, 107, 108]. In fact NRTIs have shown promising results in reducing the neuropathological consequences of HIV encephalitis in the CNS and onset of HIV-associated dementia (HAD) [108–110]. Notably, in CNS, macrophages represent the major HIV infected population [101]. In addition, NRTIs treatments in macrophages result in fewer emergences of resistant HIV mutants as compared to lymphocytes [111].
Strikingly, NRTIs efficacy is remarkably different in acutely and chronically infected macrophages. Exact mechanism responsible for such observation is poorly understood. Since chronically infected cells possess integrated HIV DNA into host chromatin, HIV RNA produced via integrated DNA using transcription by host RNA polymerase is therefore not susceptible to NRTIs. Besides this, there must be several other mechanisms responsible for the difference in the efficacy of NRTIs between chronically and acutely infected macrophages [13, 108]. Notably, NRTIs are associated with several undesirable effects including their interference with cell cycle and mitochondrial environment and also induce apoptosis [112, 113].
Non nucleoside reverse transcriptase inhibitors (NNRTIs)
Licensed NNRTIs include rilpivirine, etravirine, delavirdine, efavirenz and nevirapine. Unlike NRTIs, NNRTIs do not require phosphorylation nor compete with natural dNTPs pools for their action. NNRTIs act by binding to the hydrophobic pocket near the reverse transcriptase active site resulting in the inhibition of polymerization reaction [13, 106]. Since NNRTIs efficacy does not depend upon the cellular dNTPs pools, therefore their impact on acutely infected macrophages and CD4+ T cells is not significantly different. Furthermore, macrophage colony stimulating factor which positively regulates the dNTPs pool, have no effect on the NNRTIs efficacy against HIV [106]. Notably, NNRTIs have less adverse effects as compared to NRTIs. However, Badley research group has studied the side effects of NNRTI in Jurkat T cells and PBMCs. They observed the induction of caspase and mitochondrial dependent apoptosis by NNRTIs [114].
Like NRTIs, NNRTIs anti-HIV activities remarkably differ between acutely infected and chronically infected macrophages. To be more precise, EC50 of NNRTIs against acutely infected macrophages varies from 10 to 50nM. On the other hand, their effect is negligible against chronically infected macrophages [13, 108]. Reasons for these observations are incompletely understood.
Integrase inhibitors
Chronic HIV infection is mostly characterized by integration of proviral DNA into the host chromatin (Figure 1). This process called strand transfer is governed by HIV encoded enzyme called integrase and is indispensible for the establishment of latency [115, 116] (Figure 1). Till date three integrase inhibitors (raltegravir, elvitegravirs and dolutegravir) have been approved for clinical use. Efficacy of integrase inhibitors has been studied in MDMs and lymphocytes and showed similar results [117]. Notably, even single point mutation in integrase confers resistance against the integrase inhibitor raltegravir [118]. However, other integrase inhibitors are still effective in that situation [119]. Simultaneous targeting of multiple components of HIV is necessary to avoid emergence of resistant mutants.
Protease inhibitors (PIs)
Till date 10 protease inhibitors (PIs) have been licensed for the treatment of HIV-1 infection. Unlike reverse transcriptase inhibitors, PIs act at post integration stage of HIV-1 life cycle [106] (Figure 1). HIV protease helps in the production of infectious viral progeny. PIs bind at the active site of HIV proteases and make them non functional (Figure 1). As compared to reverse transcriptase inhibitors, PIs are effective in both acutely as well as chronically infected macrophages and CD4+ T cells. However, concentration required for effective HIV inhibition is more in case of chronically infected macrophages as compared to CD4+ T cells [120, 121]. In clinical situation, bioavailability of PIs in plasma and tissue specific macrophages is considerably different. As a result, HIV in tissue macrophages may escape from PIs [106]. Furthermore, since so far no impact of PIs on integrated HIV DNA has been reported, therefore lapse of PIs treatment will rapidly result in the production and release of infectious HIV virions [106].
Entry/fusion inhibitors
Till date, enfuvirtide and maraviroc are the two approved entry inhibitors against HIV [105]. Enfuvirtide (also called Fuzeon, T-20) is a derived from gp41 (HIV envelope protein), which inhibit hairpin formation critical for the fusion of viral envelope with host membrane [13, 106, 121] (Figure 1). Enfuvirtide inhibits HIV-1 entry into different target cells including macrophages, PBMCs and immature dendritic cells [122]. However, comprehensive studies of these inhibitors in primary macrophages are further needed.
On the other hand, maraviroc is a small molecule which binds with CCR5 receptor reversibly and prevents the virus host interactions [13] (Figure 1). Notably, maraviroc is so far the only CCR5 antagonist licensed for the treatment of HIV-infected patients [123]. Due to serious side effects and lack of clinical efficacy, other CCR5 inhibitors including aplaviroc, vicriviro and TAK-779 are no more considered for clinical development. Resistance to maraviroc has been reported [124] and responsible mechanisms have been studied [125]. New CCR5 antagonists are in different stages of development and cocktail of these CCR5 antagonists with other ART may improve the results against HIV infection.
Novel therapeutics against HIV-1 in macrophages
Multiple novel approaches are required to completely eradicate HIV-1 from infected patients. Here we will focus on novel molecular therapeutics tools emerged against HIV-1 in macrophages.
Carbohydrate-binding agents (CBAs)
CBAs have been described as anti-HIV molecules which specifically target glycans of HIV-1 gp120 [126, 127]. As a result of glycosylation of gp120, macrophages and dendritic cells lose their ability to recognize and present processed antigen to the CD4+ T cells to significant level, resulting in inefficient transfer of infection to the CD4+ T cells [13]. Balzarini and colleagues revealed that even brief exposure of HIV-1 to CBA hampers the ability of immature dendritic cells (having glycan-targeting C-type DC-SIGN lectin receptor) to bind HIV-1 and prevent syncytia formation when co-inoculated with T cells [128]. Recently, Balzarini research laboratory has shown that griffithsin (GRFT), an anti-HIV CBA inhibits the interaction between DC-SIGN and HIV gp120 protein and efficiently hampers the transfer of HIV-1 to CD4+ T cells [129]. Impact of CBAs in chronic HIV-1 infection is poorly defined.
PI3K/Akt blocking agents
The PI3K/Akt signaling cascades have been widely recognized as a favorable target for anti-cancer strategies [130]. Several groups demonstrated that PI3K/Akt inhibitors in cancer therapy are well tolerated and have minimum toxicological profile in animal models and humans [131, 132]. In past few years inhibitors of PI3K/Akt signaling have been employed as anti-HIV-1 strategy. PI3K/Akt inhibitors have been shown to effectively inhibit HIV-1 replication in acutely infected primary macrophages. PI3K/Akt inhibitors used by Chugh et al. were optimally effective at 200 nM which is far above from physiological relevant concentrations [133]. Despite this, their results provide a valuable insight into a signaling event specifically active in HIV-1 infected cells. Additionally, the blockade of the PI3K/Akt pathway could favor apoptosis and the clearance of infected cells. The impact of PI3K/Akt inhibitors on chronically infected macrophages needs to be further investigated.
Small interfering RNA (siRNA)
siRNAs are robust molecules which can practically degrade any viral RNA species [134]. siRNAs or shRNAs have been found to be effective in inhibiting HIV-1 replication in several cell types including primary macrophages [135]. Information of siRNAs against HIV has been compiled in the form of database called HIVsiDB [136]. HIVsiDB has information of more than 750 anti-HIV siRNAs [136]. In vivo toxicity, lack of effective delivery tools, generations of viral escape mutants are main hurdles in the development of siRNA as an effective therapeutic tool against HIV.
Immune based therapeutics
HIV-1 infection ultimately results in the depletion of CD4+ T and CD8+ T cells. Efforts have been made in the direction of boosting immunity against HIV-1 [137]. For example, in various studies the application of IL-2, IL7, IL-12 and growth hormone have been reported to result in increase in CD4+ T counts in HIV-1 infected individuals [138–141]. Interestingly, IL-2 along with ART significantly reduces HIV-1 replication in infected patients as compared to ART only treated patients. However, upon treatment cessation virus bounce back indicating the inability of IL-2 to enhance immunity for the longer period of time [96, 138]. In addition, role of IL-15 has been suggested in improving functionality of anti-HIV CTLs and natural killer (NK) cells in vitro[142]. Moreover, IL-15 enhances simian immunodeficiency virus (SIV) specific CD8+ T cells, NK cells and decreases the number of SIV infected cells in lymph node in infected rhesus macaque [143]. Surprisingly, viral load was found to be increased more than two fold upon IL-15 treatment [143]. Notably, IL-21 treatment in SIV infected macaques resulted in increase in granzymes B and perforins in NK cells and CD8+ T cells [96, 144]. Benefits of such transient immunity evoke by interleukins and impact of continuous use of such immune based therapeutics on the health of HIV-1 infected individuals need to be carefully addressed.
IL-27, an anti-HIV cytokine
IL-27 is a cytokine belonging to the IL-12 cytokine family and plays important roles in innate and adaptive immunity [145]. IL-27 is produced by epithelial cells, dendritic cells and macrophages [146]. Several research groups have documented the anti-HIV properties of IL-27 in MDMs, CD4+ T cells, immature and mature dendritic cells [147]. Mechanistic details of anti-HIV cytokine IL-27 have been recently revealed. IL-27 down regulates the expression of SPTBN1 (spectrin β nonerythrocyte 1), one of the host factor required for HIV-1 infection in macrophages [148]. Furthermore, IL-27 down-regulates the expression of SPTBN1 via TAK-1-mediated MAPK signaling cascade [148]. Importantly, their results indicate that SPTBN1 is a critical host component which can be targeted to inhibit HIV-1 replication in one of the principal HIV-1 reservoirs, the macrophages.
Macrophage targeted carriers
Effective therapeutic agent must be complimented with effective delivery tools for the successful delivery of results. Nanotechnology has made it possible to deliver the therapeutic agents to specific cell types or anatomical location which otherwise are not accessible by conventional delivery methods [149]. It is assumed that anti-HIV drugs delivered via nano-carrier can be selectively accumulate in infected cell types while uninfected cells will have much lower concentration of drugs therefore, will have less side effects [150]. Wan and colleagues have developed nano-carrier based system for drug delivery in macrophages using formyl methionine-leucine-phenylalanine (fMLF) peptide-PEG derivatives [151]. fMLF are employed because fMLF receptors are specifically present on phagocytic cells including macrophages and fMLF binds to the receptors present on macrophages with high affinity [151, 152]. Bio-distribution of fMLF-PEG nano-carrier was studied in vivo, revealed the greater accumulation of fMLF-PEG into macrophages of kidneys, spleen and liver as compared to only PEG [152]. Results are encouraging and suggest the feasibility of specifically targeting HIV-1 reservoir in macrophages.
Myeloid cells of central nervous system (CNS) and HIV-1
ART has significantly reduced morbidity and mortality burden associated with HIV-1. However, despite of that significant number of the patients receiving ART develops HIV-1 associated CNS disorders [153, 154]. Notably, Zink and colleagues demonstrated that ART is able to reduce the viral load in cerebrospinal fluid of macaques infected with simian immunodeficiency virus (SIV). However, they observed the presence of SIV DNA in CNS [155]. In CNS, major reservoirs of HIV-1 are the cells of myeloid origin which include meningeal macrophages, microglia and perivascular cells. Therefore, the interplay between these cells and HIV-1 is of utmost importance. Recently role of HIV-1 Tat protein has been shown in disrupting synaptical architecture in vitro as well in vivo[156–158]. Lu and colleagues have further demonstrated the involvement of CNS resident myeloid cells in deteriorating the synaptical architecture in response to Tat [157].
In addition, recently role of cathepsin B secreted by HIV-1 infected macrophages in neural apoptosis has been also described [159]. Notably, low level of cathepsin B has been detected in the post-mortem brain tissue of HIV-1 individual with HAD but not in normal individual or HIV-1 infected individual with normal cognition. Their results suggest the involvement of cathepsin B in HAD [159]. Altogether above findings provide a valuable insight into the mechanism of HIV-1 associated CNS disorder which involves myeloid cells, their secretome and viral proteins. These novel findings will help in generating new targets for managing HAD.
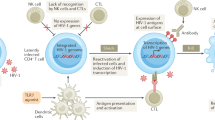
HIV-1 latency in macrophages and reactivation: the “flushing out” therapy
Although highly active retroviral therapy (ART) has significantly reduced viral levels (50 copies/ml) in infected patients however, interruption of ART results in rapid increase in viremia. HIV infection leads to the rapid depletion of CD4+ T and CD8+ T cells. Despite there is certain percent of cells where virus integrate with host chromatin. These cells do not produce virus in resting condition, however produce it upon activation [160, 161]. These cells represent a pool of latent infection and are a main obstacle in complete eradication of HIV-1 from infected patients [96, 116, 162]. Besides resting CD4+ T cells, it is suggested that monocytes, macrophages, dendritic cells and hematopoietic stem cells can be latently infected with HIV [163–165]. There are experimental evidences in the support of latency in monocytes [163, 166].
Role of macrophages in dissemination of virus and expanding viral reservoir especially in T lymphocytes has been discussed elsewhere in this review. Prolonged life span and resistance to HIV cytopathic effects make macrophages as unique viral reservoirs. However, association between HIV-1 latency and macrophages is less clear. HIV infected patients on ART treatment are reported to have only few macrophages infected in lymph nodes however undergoes reactivation in case of opportunistic infections [167]. Interestingly, FDA approved amphotericin B (an antifungal drug) has been reported to reactivate HIV-1 in THP89GFP cells (a model cell line for the HIV-1 latency in macrophages) but not in T lymphocytes [168]. However, when amphotericin B induced THP89GFP cells are co-cultured with J89GFP (latently infected T cells), they activate latent HIV in latter cells [168]. In addition, recently role of polybacterial challenge in activating latent HIV-1 in the cells of monocyte/macrophage lineage has been shown in vitro[169, 170]. These findings indicate that macrophages may be a site of HIV-1 latent infection. Unlike CD4+ T cells, pre-integration latency in macrophages may contribute to the viral reservoir formation to a significant extent [171]. Mechanism/s responsible for post integration latency in macrophages is poorly understood. However, presence of host transcriptional repressors, anti-HIV microRNA and lack of functional Tat could play significant role in establishing post-integration latency in infected macrophage [171]. For example host factor C/EBPb is known to repressor HIV-1 transcription in macrophages which may contribute to HIV latency. In addition, in human microglial cells, CTIP2 (a highly expressed transcriptional repressor in brain) is known to inhibit the HIV-1 replication mediated by recruitment of chromatin modifying complex involving HDAC1, HDAC2 and methylase SUV39H1 [172]. Role of CTIP2 has been suggested in post integration latency in microglia cells [165, 172].
Current efforts have been made in the direction of reactivation of HIV from latent reservoir followed by their complete removal by ART [96]. According to this hypothesis, cells in which latency is reactivated should die either due to viral cytopathic effect or due to recognition by cytotoxic T cells [96, 115]. Furthermore, the fresh infection by viral progeny (released from lysed cells) will be inhibited by ART.
Several kinds of new approaches have been employed in reactivating HIV including the use of histone deacetylase inhibitors (HDACi) such as valproic acid (VPA), trichostatin (TSA), suberoylanilide hydroxyamic acid (SAHA) and sodium butyrate, methylation inhibitors including BIX-01294, 5-aza-2′deoxycytidine (Aza-CdR) and chaetocin, NFκB activators for example TNF-α and bryostatin and protein kinase C modulators and immune modulators including IL-7 and IL-15 [96, 105, 116, 173]. These new compounds have shown significant results in reactivating latency in CD4+ T cells and are at different stages of development. For example first successful clinical trial has been reported with HDACi, valproic acid (VPA) [165, 174]. However, these findings are not confirmed in other trials [175, 176].
Regarding efficacy of these novel compounds in reactivating latency in macrophages, not many reports are available. However, several HDACi have been tested in ACH2 and U1 cell lines and found to be equally effective in both cell lines [177]. Recently, Matalon and colleague tested ITF2357 (givinostat) and VPA in ACH2 and U1 cell line. Their data revealed that ITF2357 is more potent in activating latency as compared to VPA [178]. Notably, givinostat has been found to be safe in healthy individuals in phase I trial [179]. Altogether data from in vitro studies suggest that agents used in reactivating latency in T cells have similar effects in cells of monocyte/macrophage lineage. However, in clinical trials viral load has been mainly determined in T lymphocytes. Importantly, isolation of monocytes followed by production of monocyte derived macrophages is rather a lengthy process as compared to isolation of T lymphocytes. In addition, brain resident macrophages represent the anatomical sanctuaries where drug penetration is poor and determination of drug efficacy in these sanctuaries is rather a difficult task [180, 181]. Furthermore, the presence of efflux pumps and array of metabolic enzymes in blood brain barrier further put the efficacy of drugs in a difficult proposition. CNS resident macrophages play an important role in HAD, a severe morbidity of HIV-1 infection. Treating HIV-1 needs holistic view where besides T lymphocytes cells of monocyte/macrophage lineage must be taken into consideration. Ignoring one or other viral reservoir will not result in any favorable outcome.
Conclusion
Macrophages are among the early targets of HIV-1. They also act as chronic and latent viral reservoirs. Although ART has suppressed viremia in most of infected patients, complete eradication is not possible without clearance of HIV-1 from latent reservoirs. Novel therapeutics options have emerged against these reservoirs. However, delivery of therapeutic molecules in vivo is still a major challenge. In the future, combinatorial therapies equipped with precise delivery tools can fulfill the scientific dream of the complete eradication of HIV-1 from infected patients.
References
Iordanskiy S, Santos S, Bukrinsky M: Nature, nurture and HIV: the effect of producer cell on viral physiology. Virology. 2013, 443: 208-213. 10.1016/j.virol.2013.05.023.
Abbas W, Herbein G: T-cell signaling in HIV-1 infection. Open Virol J. 2013, 7: 57-71. 10.2174/1874357920130621001.
Ackerman AL, Cresswell P: Cellular mechanisms governing cross-presentation of exogenous antigens. Nat Immunol. 2004, 5: 678-684.
Koppensteiner H, Brack-Werner R, Schindler M: Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology. 2012, 9: 82-10.1186/1742-4690-9-82.
Crowe SM, Mills J, Kirihara J, Boothman J, Marshall JA, McGrath MS: Full-length recombinant CD4 and recombinant gp120 inhibit fusion between HIV infected macrophages and uninfected CD4-expressing T-lymphoblastoid cells. AIDS Res Hum Retroviruses. 1990, 6: 1031-1037.
Crowe SM, Mills J, Elbeik T, Lifson JD, Kosek J, Marshall JA, Engleman EG, McGrath MS: Human immunodeficiency virus-infected monocyte-derived macrophages express surface gp120 and fuse with CD4 lymphoid cells in vitro: a possible mechanism of T lymphocyte depletion in vivo. Clin Immunol Immunopathol. 1992, 65: 143-151. 10.1016/0090-1229(92)90217-C.
Groot F, Welsch S, Sattentau QJ: Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008, 111: 4660-4663. 10.1182/blood-2007-12-130070.
Badley AD, Dockrell D, Simpson M, Schut R, Lynch DH, Leibson P, Paya CV: Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J Exp Med. 1997, 185: 55-64. 10.1084/jem.185.1.55.
Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O'Brien WA, Verdin E: Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998, 395: 189-194. 10.1038/26026.
Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS, Meltzer MS: Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988, 167: 1428-1441. 10.1084/jem.167.4.1428.
Carter CA, Ehrlich LS: Cell biology of HIV-1 infection of macrophages. Annu Rev Microbiol. 2008, 62: 425-443. 10.1146/annurev.micro.62.081307.162758.
Kelly J, Beddall MH, Yu D, Iyer SR, Marsh JW, Wu Y: Human macrophages support persistent transcription from unintegrated HIV-1 DNA. Virology. 2008, 372: 300-312. 10.1016/j.virol.2007.11.007.
Gavegnano C, Schinazi RF: Antiretroviral therapy in macrophages: implication for HIV eradication. Antivir Chem Chemother. 2009, 20: 63-78. 10.3851/IMP1374.
Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA: Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998, 393: 648-659. 10.1038/31405.
Herbein G, Varin A: The macrophage in HIV-1 infection: from activation to deactivation?. Retrovirology. 2010, 7: 33-10.1186/1742-4690-7-33.
Zaitseva M, Blauvelt A, Lee S, Lapham CK, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H: Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997, 3: 1369-1375. 10.1038/nm1297-1369.
Yi Y, Rana S, Turner JD, Gaddis N, Collman RG: CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J Virol. 1998, 72: 772-777.
Abbas W, Herbein G: Plasma membrane signaling in HIV-1 infection. Biochim Biophys Acta. 2013, doi:10.1016/j.bbamem.2013.06.020
Mougel M, Houzet L, Darlix JL: When is it time for reverse transcription to start and go?. Retrovirology. 2009, 6: 24-10.1186/1742-4690-6-24.
Herbein G, Gras G, Khan KA, Abbas W: Macrophage signaling in HIV-1 infection. Retrovirology. 2010, 7: 34-10.1186/1742-4690-7-34.
Gavegnano C, Kennedy EM, Kim B, Schinazi RF: The impact of macrophage nucleotide pools on HIV-1 reverse transcription, viral replication, and the development of novel antiviral agents. Mol Biol Int. 2012, 2012: 625983-
Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M: Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol. 1998, 72: 4633-4642.
Furge LL, Guengerich FP: Analysis of nucleotide insertion and extension at 8-oxo-7,8-dihydroguanine by replicative T7 polymerase exo- and human immunodeficiency virus-1 reverse transcriptase using steady-state and pre-steady-state kinetics. Biochemistry. 1997, 36: 6475-6487. 10.1021/bi9627267.
Diamond TL, Roshal M, Jamburuthugoda VK, Reynolds HM, Merriam AR, Lee KY, Balakrishnan M, Bambara RA, Planelles V, Dewhurst S, Kim B: Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J Biol Chem. 2004, 279: 51545-51553. 10.1074/jbc.M408573200.
Sheehy AM, Gaddis NC, Choi JD, Malim MH: Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002, 418: 646-650. 10.1038/nature00939.
Neil SJ, Zang T, Bieniasz PD: Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008, 451: 425-430. 10.1038/nature06553.
Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J: Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011, 474: 658-661. 10.1038/nature10195.
Vicenzi E, Poli G: Novel factors interfering with human immunodeficiency virus-type 1 replication in vivo and in vitro. Tissue Antigens. 2013, 81: 61-71. 10.1111/tan.12047.
Goila-Gaur R, Strebel K: HIV-1 Vif, APOBEC, and intrinsic immunity. Retrovirology. 2008, 5: 51-10.1186/1742-4690-5-51.
Sauter D, Kirchhoff F: Tetherin antagonism by primate lentiviral nef proteins. Curr HIV Res. 2011, 9: 514-523. 10.2174/157016211798842044.
Zhang F, Wilson SJ, Landford WC, Virgen B, Gregory D, Johnson MC, Munch J, Kirchhoff F, Bieniasz PD, Hatziioannou T: Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe. 2009, 6: 54-67. 10.1016/j.chom.2009.05.008.
Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T, Pancino G, Priet S, Canard B, Laguette N, Benkirane M, Transy C, Landau NR, Kim B, Margottin-Goguet F: SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol. 2012, 13: 223-228. 10.1038/ni.2236.
Liu L, Oliveira NM, Cheney KM, Pade C, Dreja H, Bergin AM, Borgdorff V, Beach DH, Bishop CL, Dittmar MT, McKnight A: A whole genome screen for HIV restriction factors. Retrovirology. 2011, 8: 94-10.1186/1742-4690-8-94.
Allouch A, David A, Amie SM, Lahouassa H, Chartier L, Margottin-Goguet F, Barré-Sinoussi F, Kim B, Sáez-Cirión A, Pancino G: p21-mediated RNR2 repression restricts HIV-1 replication in macrophages by inhibiting dNTP biosynthesis pathway. Proc Natl Acad Sci U S A. 2013, 110: E3997-E4006. 10.1073/pnas.1306719110.
Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, Stevenson M: Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci U S A. 1992, 89: 6580-6584. 10.1073/pnas.89.14.6580.
Bukrinsky MI, Sharova N, McDonald TL, Pushkarskaya T, Tarpley WG, Stevenson M: Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci U S A. 1993, 90: 6125-6129. 10.1073/pnas.90.13.6125.
Gallay P, Stitt V, Mundy C, Oettinger M, Trono D: Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996, 70: 1027-1032.
Gallay P, Hope T, Chin D, Trono D: HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci U S A. 1997, 94: 9825-9830. 10.1073/pnas.94.18.9825.
Nitahara-Kasahara Y, Kamata M, Yamamoto T, Zhang X, Miyamoto Y, Muneta K, Iijima S, Yoneda Y, Tsunetsugu-Yokota Y, Aida Y: Novel nuclear import of Vpr promoted by importin alpha is crucial for human immunodeficiency virus type 1 replication in macrophages. J Virol. 2007, 81: 5284-5293. 10.1128/JVI.01928-06.
Jacque JM, Stevenson M: The inner-nuclear-envelope protein emerin regulates HIV-1 infectivity. Nature. 2006, 441: 641-645. 10.1038/nature04682.
Kobiler O, Drayman N, Butin-Israeli V, Oppenheim A: Virus strategies for passing the nuclear envelope barrier. Nucleus. 2012, 3: 526-539. 10.4161/nucl.21979.
Shun MC, Daigle JE, Vandegraaff N, Engelman A: Wild-type levels of human immunodeficiency virus type 1 infectivity in the absence of cellular emerin protein. J Virol. 2007, 81: 166-172. 10.1128/JVI.01953-06.
Cobos-Jimenez V, Booiman T, Hamann J, Kootstra NA: Macrophages and HIV-1. Curr Opin HIV AIDS. 2011, 6: 385-390. 10.1097/COH.0b013e3283497203.
Kilareski EM, Shah S, Nonnemacher MR, Wigdahl B: Regulation of HIV-1 transcription in cells of the monocyte-macrophage lineage. Retrovirology. 2009, 6: 118-10.1186/1742-4690-6-118.
Henderson AJ, Calame KL: CCAAT/enhancer binding protein (C/EBP) sites are required for HIV-1 replication in primary macrophages but not CD4(+) T cells. Proc Natl Acad Sci U S A. 1997, 94: 8714-8719. 10.1073/pnas.94.16.8714.
Frank I, Stoiber H, Godar S, Stockinger H, Steindl F, Katinger HW, Dierich MP: Acquisition of host cell-surface-derived molecules by HIV-1. AIDS. 1996, 10: 1611-1620. 10.1097/00002030-199612000-00004.
Tan J, Sattentau QJ: The HIV-1-containing macrophage compartment: a perfect cellular niche?. Trends Microbiol. 2013, 21: 405-412. 10.1016/j.tim.2013.05.001.
Orenstein JM, Meltzer MS, Phipps T, Gendelman HE: Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J Virol. 1988, 62: 2578-2586.
Raposo G, Moore M, Innes D, Leijendekker R, Leigh-Brown A, Benaroch P, Geuze H: Human macrophages accumulate HIV-1 particles in MHC II compartments. Traffic. 2002, 3: 718-729. 10.1034/j.1600-0854.2002.31004.x.
Pelchen-Matthews A, Kramer B, Marsh M: Infectious HIV-1 assembles in late endosomes in primary macrophages. J Cell Biol. 2003, 162: 443-455. 10.1083/jcb.200304008.
Deneka M, Pelchen-Matthews A, Byland R, Ruiz-Mateos E, Marsh M: In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J Cell Biol. 2007, 177: 329-341. 10.1083/jcb.200609050.
Kramer B, Pelchen-Matthews A, Deneka M, Garcia E, Piguet V, Marsh M: HIV interaction with endosomes in macrophages and dendritic cells. Blood Cells Mol Dis. 2005, 35: 136-142. 10.1016/j.bcmd.2005.06.006.
Welsch S, Groot F, Krausslich HG, Keppler OT, Sattentau QJ: Architecture and regulation of the HIV-1 assembly and holding compartment in macrophages. J Virol. 2011, 85: 7922-7927. 10.1128/JVI.00834-11.
Welsch S, Keppler OT, Habermann A, Allespach I, Krijnse-Locker J, Krausslich HG: HIV-1 buds predominantly at the plasma membrane of primary human macrophages. PLoS Pathog. 2007, 3: e36-10.1371/journal.ppat.0030036.
Buonaguro L, Barillari G, Chang HK, Bohan CA, Kao V, Morgan R, Gallo RC, Ensoli B: Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J Virol. 1992, 66: 7159-7167.
Ott M, Emiliani S, Van Lint C, Herbein G, Lovett J, Chirmule N, McCloskey T, Pahwa S, Verdin E: Immune hyperactivation of HIV-1-infected T cells mediated by Tat and the CD28 pathway. Science. 1997, 275: 1481-1485. 10.1126/science.275.5305.1481.
Jeang KT, Xiao H, Rich EA: Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J Biol Chem. 1999, 274: 28837-28840. 10.1074/jbc.274.41.28837.
Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC: Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993, 67: 277-287.
Huang L, Bosch I, Hofmann W, Sodroski J, Pardee AB: Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains. J Virol. 1998, 72: 8952-8960.
Albini A, Benelli R, Giunciuglio D, Cai T, Mariani G, Ferrini S, Noonan DM: Identification of a novel domain of HIV tat involved in monocyte chemotaxis. J Biol Chem. 1998, 273: 15895-15900. 10.1074/jbc.273.26.15895.
Campbell GR, Loret EP: What does the structure-function relationship of the HIV-1 Tat protein teach us about developing an AIDS vaccine?. Retrovirology. 2009, 6: 50-10.1186/1742-4690-6-50.
Chen P, Mayne M, Power C, Nath A: The Tat protein of HIV-1 induces tumor necrosis factor-alpha production. Implications for HIV-1-associated neurological diseases. J Biol Chem. 1997, 272: 22385-22388. 10.1074/jbc.272.36.22385.
Mayne M, Holden CP, Nath A, Geiger JD: Release of calcium from inositol 1,4,5-trisphosphate receptor-regulated stores by HIV-1 Tat regulates TNF-alpha production in human macrophages. J Immunol. 2000, 164: 6538-6542.
Nath A: Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002, 186 (Suppl 2): S193-S198.
Subbramanian RA, Kessous-Elbaz A, Lodge R, Forget J, Yao XJ, Bergeron D, Cohen EA: Human immunodeficiency virus type 1 Vpr is a positive regulator of viral transcription and infectivity in primary human macrophages. J Exp Med. 1998, 187: 1103-1111. 10.1084/jem.187.7.1103.
Jacquot G, Le RE, David A, Mazzolini J, Bouchet J, Bouaziz S, Niedergang F, Pancino G, Benichou S: Localization of HIV-1 Vpr to the nuclear envelope: impact on Vpr functions and virus replication in macrophages. Retrovirology. 2007, 4: 84-10.1186/1742-4690-4-84.
Bukrinsky M, Adzhubei A: Viral protein R of HIV-1. Rev Med Virol. 1999, 9: 39-49. 10.1002/(SICI)1099-1654(199901/03)9:1<39::AID-RMV235>3.0.CO;2-3.
Varin A, Decrion AZ, Sabbah E, Quivy V, Sire J, Van Lint C, Roques BP, Aggarwal BB, Herbein G: Synthetic Vpr protein activates activator protein-1, c-Jun N-terminal kinase, and NF-kappaB and stimulates HIV-1 transcription in promonocytic cells and primary macrophages. J Biol Chem. 2005, 280: 42557-42567. 10.1074/jbc.M502211200.
Eckstein DA, Sherman MP, Penn ML, Chin PS, De Noronha CM, Greene WC, Goldsmith MA: HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells. J Exp Med. 2001, 194: 1407-1419. 10.1084/jem.194.10.1407.
Vazquez N, Greenwell-Wild T, Marinos NJ, Swaim WD, Nares S, Ott DE, Schubert U, Henklein P, Orenstein JM, Sporn MB, Wahl SM: Human immunodeficiency virus type 1-induced macrophage gene expression includes the p21 gene, a target for viral regulation. J Virol. 2005, 79: 4479-4491. 10.1128/JVI.79.7.4479-4491.2005.
Das SR, Jameel S: Biology of the HIV Nef protein. Indian J Med Res. 2005, 121: 315-332.
Lamers SL, Fogel GB, Singer EJ, Salemi M, Nolan DJ, Huysentruyt LC, McGrath MS: HIV-1 Nef in macrophage-mediated disease pathogenesis. Int Rev Immunol. 2012, 31: 432-450. 10.3109/08830185.2012.737073.
Lama J, Mangasarian A, Trono D: Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr Biol. 1999, 9: 622-631. 10.1016/S0960-9822(99)80284-X.
Foster JL, Garcia JV: HIV-1 Nef: at the crossroads. Retrovirology. 2008, 5: 84-10.1186/1742-4690-5-84.
Tachado SD, Li X, Swan K, Patel N, Koziel H: Constitutive activation of phosphatidylinositol 3-kinase signaling pathway down-regulates TLR4-mediated tumor necrosis factor-alpha release in alveolar macrophages from asymptomatic HIV-positive persons in vitro. J Biol Chem. 2008, 283: 33191-33198. 10.1074/jbc.M805067200.
Lama J: The physiological relevance of CD4 receptor down-modulation during HIV infection. Curr HIV Res. 2003, 1: 167-184. 10.2174/1570162033485276.
Mangino G, Percario ZA, Fiorucci G, Vaccari G, Acconcia F, Chiarabelli C, Leone S, Noto A, Horenkamp FA, Manrique S, Romeo G, Polticelli F, Geyer M, Affabris E: HIV-1 Nef induces proinflammatory state in macrophages through its acidic cluster domain: involvement of TNF alpha receptor associated factor 2. PLoS One. 2011, 6: e22982-10.1371/journal.pone.0022982.
Mangino G, Percario ZA, Fiorucci G, Vaccari G, Manrique S, Romeo G, Federico M, Geyer M, Affabris E: In vitro treatment of human monocytes/macrophages with myristoylated recombinant Nef of human immunodeficiency virus type 1 leads to the activation of mitogen-activated protein kinases, IkappaB kinases, and interferon regulatory factor 3 and to the release of beta interferon. J Virol. 2007, 81: 2777-2791. 10.1128/JVI.01640-06.
Khan KA, Abbas W, Varin A, Kumar A, Di Martino V, Dichamp I, Herbein G: HIV-1 Nef interacts with HCV core, recruits TRAF2, TRAF5 and TRAF6, and stimulates HIV-1 replication in macrophages. J Innate Immun. 2013, 5: 639-656. 10.1159/000350517.
Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A: Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995, 1: 129-134. 10.1038/nm0295-129.
Swingler S, Brichacek B, Jacque JM, Ulich C, Zhou J, Stevenson M: HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature. 2003, 424: 213-219. 10.1038/nature01749.
Oyaizu N, Adachi Y, Hashimoto F, McCloskey TW, Hosaka N, Kayagaki N, Yagita H, Pahwa S: Monocytes express Fas ligand upon CD4 cross-linking and induce CD4+ T cells apoptosis: a possible mechanism of bystander cell death in HIV infection. J Immunol. 1997, 158: 2456-2463.
Geleziunas R, Xu W, Takeda K, Ichijo H, Greene WC: HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature. 2001, 410: 834-838. 10.1038/35071111.
Mahlknecht U, Deng C, Lu MC, Greenough TC, Sullivan JL, O’Brien WA, Herbein G: Resistance to apoptosis in HIV-infected CD4+ T lymphocytes is mediated by macrophages: role for Nef and immune activation in viral persistence. J Immunol. 2000, 165: 6437-6446.
Zhang M, Li X, Pang X, Ding L, Wood O, Clouse K, Hewlett I, Dayton AI: Identification of a potential HIV-induced source of bystander-mediated apoptosis in T cells: upregulation of trail in primary human macrophages by HIV-1 tat. J Biomed Sci. 2001, 8: 290-296. 10.1007/BF02256603.
Yang Y, Tikhonov I, Ruckwardt TJ, Djavani M, Zapata JC, Pauza CD, Salvato MS: Monocytes treated with human immunodeficiency virus Tat kill uninfected CD4(+) cells by a tumor necrosis factor-related apoptosis-induced ligand-mediated mechanism. J Virol. 2003, 77: 6700-6708. 10.1128/JVI.77.12.6700-6708.2003.
Lin RH, Hwang YW, Yang BC, Lin CS: TNF receptor-2-triggered apoptosis is associated with the down-regulation of Bcl-xL on activated T cells and can be prevented by CD28 costimulation. J Immunol. 1997, 158: 598-603.
Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD: Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994, 68: 4650-4655.
Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, Segal JP, Cao Y, Rowland-Jones SL, Cerundolo V, Hurley A, Markowitz M, Ho DD, Nixon DF, McMichael AJ: Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998, 279: 2103-2106. 10.1126/science.279.5359.2103.
Pozzi LA, Maciaszek JW, Rock KL: Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells. J Immunol. 2005, 175: 2071-2081.
Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D: HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998, 391: 397-401. 10.1038/34929.
Fujiwara M, Takiguchi M: HIV-1-specific CTLs effectively suppress replication of HIV-1 in HIV-1-infected macrophages. Blood. 2007, 109: 4832-4838. 10.1182/blood-2006-07-037481.
Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM: M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000, 164: 6166-6173.
Cassol E, Cassetta L, Rizzi C, Alfano M, Poli G: M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J Immunol. 2009, 182: 6237-6246. 10.4049/jimmunol.0803447.
Badley AD, Pilon AA, Landay A, Lynch DH: Mechanisms of HIV-associated lymphocyte apoptosis. Blood. 2000, 96: 2951-2964.
Badley AD, Sainski A, Wightman F, Lewin SR: Altering cell death pathways as an approach to cure HIV infection. Cell Death Dis. 2013, 4: e718-10.1038/cddis.2013.248.
Schnepple DJ, Shepard B, Bren GD, Cummins NW, Natesampillai S, Trushin S, Algeciras-Schimnich A, Meng XW, Sainski AM, Rizza SA, Kaufmann SH, Badley AD: Isolation of a TRAIL antagonist from the serum of HIV-infected patients. J Biol Chem. 2011, 286: 35742-35754. 10.1074/jbc.M111.274639.
Sainski AM, Natesampillai S, Cummins NW, Bren GD, Taylor J, Saenz DT, Poeschla EM, Badley AD: The HIV-1-specific protein Casp8p41 induces death of infected cells through Bax/Bak. J Virol. 2011, 85: 7965-7975. 10.1128/JVI.02515-10.
Cummins NW, Badley AD: Anti-apoptotic mechanisms of HIV: lessons and novel approaches to curing HIV. Cell Mol Life Sci. 2013, 70: 3355-3363. 10.1007/s00018-012-1239-3.
Swingler S, Mann AM, Zhou J, Swingler C, Stevenson M: Apoptotic killing of HIV-1-infected macrophages is subverted by the viral envelope glycoprotein. PLoS Pathog. 2007, 3: 1281-1290.
Busca A, Saxena M, Kumar A: Critical role for antiapoptotic Bcl-xL and Mcl-1 in human macrophage survival and cellular IAP1/2 (cIAP1/2) in resistance to HIV-Vpr-induced apoptosis. J Biol Chem. 2012, 287: 15118-15133. 10.1074/jbc.M111.312660.
Pomerantz RJ, Horn DL: Twenty years of therapy for HIV-1 infection. Nat Med. 2003, 9: 867-873. 10.1038/nm0703-867.
Anderson AM, Lennox JL: Antiretroviral therapy: when to start and which drugs to use. Curr Infect Dis Rep. 2008, 10: 332-339. 10.1007/s11908-008-0053-4.
Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD: Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998, 338: 853-860. 10.1056/NEJM199803263381301.
Abbas W, Herbein G: Molecular understanding of HIV-1 latency. Adv Virol. 2012, 2012: 574967-
Perno CF, Svicher V, Schols D, Pollicita M, Balzarini J, Aquaro S: Therapeutic strategies towards HIV-1 infection in macrophages. Antiviral Res. 2006, 71: 293-300. 10.1016/j.antiviral.2006.05.015.
Aquaro S, Calio R, Balestra E, Bagnarelli P, Cenci A, Bertoli A, Tavazzi B, Di Pierro D, Francesconi M, Abdelahad D, Perno CF: Clinical implications of HIV dynamics and drug resistance in macrophages. J Biol Regul Homeost Agents. 1998, 12: 23-27.
Aquaro S, Svicher V, Schols D, Pollicita M, Antinori A, Balzarini J, Perno CF: Mechanisms underlying activity of antiretroviral drugs in HIV-1-infected macrophages: new therapeutic strategies. J Leukoc Biol. 2006, 80: 1103-1110.
Haworth SJ, Christofalo B, Anderson RD, Dunkle LM: A single-dose study to assess the penetration of stavudine into human cerebrospinal fluid in adults. J Acquir Immune Defic Syndr Hum Retrovirol. 1998, 17: 235-238. 10.1097/00042560-199803010-00008.
Limoges J, Persidsky Y, Poluektova L, Rasmussen J, Ratanasuwan W, Zelivyanskaya M, McClernon DR, Lanier ER, Gendelman HE: Evaluation of antiretroviral drug efficacy for HIV-1 encephalitis in SCID mice. Neurology. 2000, 54: 379-389. 10.1212/WNL.54.2.379.
Aquaro S, Svicher V, Ceccherini-Silberstein F, Cenci A, Marcuccilli F, Giannella S, Marcon L, Caliò R, Balzarini J, Perno CF: Limited development and progression of resistance of HIV-1 to the nucleoside analogue reverse transcriptase inhibitor lamivudine in human primary macrophages. J Antimicrob Chemother. 2005, 55: 872-878. 10.1093/jac/dki104.
Chariot P, Monnet I, Gherardi R: Cytochrome c oxidase reaction improves histopathological assessment of zidovudine myopathy. Ann Neurol. 1993, 34: 561-565. 10.1002/ana.410340409.
Viora M, Di GG, Rivabene R, Malorni W, Fattorossi A: Interference with cell cycle progression and induction of apoptosis by dideoxynucleoside analogs. Int J Immunopharmacol. 1997, 19: 311-321. 10.1016/S0192-0561(97)00041-6.
Pilon AA, Lum JJ, Sanchez-Dardon J, Phenix BN, Douglas R, Badley AD: Induction of apoptosis by a nonnucleoside human immunodeficiency virus type 1 reverse transcriptase inhibitor. Antimicrob Agents Chemother. 2002, 46: 2687-2691. 10.1128/AAC.46.8.2687-2691.2002.
Siliciano RF, Greene WC: HIV latency. Cold Spring Harb Perspect Med. 2011, 1: a007096-
Van Lint C, Bouchat S, Marcello A: HIV-1 transcription and latency: an update. Retrovirology. 2013, 10: 67-10.1186/1742-4690-10-67.
Scopelliti F, Pollicita M, Ceccherini-Silberstein F, Di SF, Surdo M, Aquaro S, Perno CF: Comparative antiviral activity of integrase inhibitors in human monocyte-derived macrophages and lymphocytes. Antiviral Res. 2011, 92: 255-261. 10.1016/j.antiviral.2011.08.008.
Marsden MD, Avancena P, Kitchen CM, Hubbard T, Zack JA: Single mutations in HIV integrase confer high-level resistance to raltegravir in primary human macrophages. Antimicrob Agents Chemother. 2011, 55: 3696-3702. 10.1128/AAC.00566-11.
Canducci F, Ceresola ER, Saita D, Castagna A, Gianotti N, Underwood M, Burioni R, Lazzarin A, Clementi M: In vitro phenotypes to elvitegravir and dolutegravir in primary macrophages and lymphocytes of clonal recombinant viral variants selected in patients failing raltegravir. J Antimicrob Chemother. 2013, 68: 2525-2532. 10.1093/jac/dkt220.
Perno CF, Aquaro S, Rosenwirth B, Balestra E, Peichl P, Billich A, Villani N, Caliò R: In vitro activity of inhibitors of late stages of the replication of HIV in chronically infected macrophages. J Leukoc Biol. 1994, 56: 381-386.
Perno CF, Newcomb FM, Davis DA, Aquaro S, Humphrey RW, Calio R, Yarchoan R: Relative potency of protease inhibitors in monocytes/macrophages acutely and chronically infected with human immunodeficiency virus. J Infect Dis. 1998, 178: 413-422. 10.1086/515642.
Yi Y, Loftin L, Wang L, Ratcliffe SJ, Isaacman-Beck J, Collman RG: Entry coreceptor use and fusion inhibitor T20 sensitivity of dual-tropic R5X4 HIV-1 in primary macrophage infection. J Acquir Immune Defic Syndr. 2008, 47: 285-292. 10.1097/QAI.0b013e31816520f6.
Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M: Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005, 49: 4721-4732. 10.1128/AAC.49.11.4721-4732.2005.
Moore JP, Kuritzkes DR: A piece de resistance: how HIV-1 escapes small molecule CCR5 inhibitors. Curr Opin HIV AIDS. 2009, 4: 118-124. 10.1097/COH.0b013e3283223d46.
Anastassopoulou CG, Ketas TJ, Klasse PJ, Moore JP: Resistance to CCR5 inhibitors caused by sequence changes in the fusion peptide of HIV-1 gp41. Proc Natl Acad Sci U S A. 2009, 106: 5318-5323. 10.1073/pnas.0811713106.
Balzarini J: Carbohydrate-binding agents: a potential future cornerstone for the chemotherapy of enveloped viruses?. Antivir Chem Chemother. 2007, 18: 1-11.
Pollicita M, Schols D, Aquaro S, Peumans WJ, Van Damme EJ, Perno CF, Balzarini J: Carbohydrate-binding agents (CBAs) inhibit HIV-1 infection in human primary monocyte-derived macrophages (MDMs) and efficiently prevent MDM-directed viral capture and subsequent transmission to CD4+ T lymphocytes. Virology. 2008, 370: 382-391. 10.1016/j.virol.2007.08.033.
Balzarini J, Van HY, Vermeire K, Vanham G, Schols D: Carbohydrate-binding agents efficiently prevent dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-directed HIV-1 transmission to T lymphocytes. Mol Pharmacol. 2007, 71: 3-11.
Hoorelbeke B, Xue J, LiWang PJ, Balzarini J: Role of the carbohydrate-binding sites of griffithsin in the prevention of DC-SIGN-mediated capture and transmission of HIV-1. PLoS One. 2013, 8: e64132-10.1371/journal.pone.0064132.
El-Deiry WS: Akt takes centre stage in cell-cycle deregulation. Nat Cell Biol. 2001, 3: E71-E73. 10.1038/35060148.
Morgensztern D, McLeod HL: PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005, 16: 797-803. 10.1097/01.cad.0000173476.67239.3b.
Knowling M, Blackstein M, Tozer R, Bramwell V, Dancey J, Dore N, Matthews S, Eisenhauer E: A phase II study of perifosine (D-21226) in patients with previously untreated metastatic or locally advanced soft tissue sarcoma: a National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2006, 24: 435-439. 10.1007/s10637-006-6406-7.
Chugh P, Bradel-Tretheway B, Monteiro-Filho CM, Planelles V, Maggirwar SB, Dewhurst S, Kim B: Akt inhibitors as an HIV-1 infected macrophage-specific anti-viral therapy. Retrovirology. 2008, 5: 11-10.1186/1742-4690-5-11.
Leonard JN, Schaffer DV: Antiviral RNAi therapy: emerging approaches for hitting a moving target. Gene Ther. 2006, 13: 532-540. 10.1038/sj.gt.3302645.
Song E, Lee SK, Dykxhoorn DM, Novina C, Zhang D, Crawford K, Cerny J, Sharp PA, Lieberman J, Manjunath N, Shankar P: Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J Virol. 2003, 77: 7174-7181. 10.1128/JVI.77.13.7174-7181.2003.
Tyagi A, Ahmed F, Thakur N, Sharma A, Raghava GP, Kumar M: HIVsirDB: a database of HIV inhibiting siRNAs. PLoS One. 2011, 6: e25917-10.1371/journal.pone.0025917.
Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, Chomont N, Chun TW, Churchill M, Di Mascio M, Katlama C, Lafeuillade A, Landay A, Lederman M, Lewin SR, Maldarelli F, Margolis D, Markowitz M, Martinez-Picado J, Mullins JI, Mellors J, Moreno S, O'Doherty U, Palmer S, Penicaud MC, Peterlin M, Poli G, Routy JP, Rouzioux C, Silvestri G, Stevenson M: Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012, 12: 607-614. 10.1038/nri3262.
Chun TW, Davey RT, Engel D, Lane HC, Fauci AS: Re-emergence of HIV after stopping therapy. Nature. 1999, 401: 874-875. 10.1038/44755.
Jacobson MA, Hardy D, Connick E, Watson J, DeBruin M: Phase 1 trial of a single dose of recombinant human interleukin-12 in human immunodeficiency virus-infected patients with 100–500 CD4 cells/microL. J Infect Dis. 2000, 182: 1070-1076. 10.1086/315819.
Napolitano LA, Lo JC, Gotway MB, Mulligan K, Barbour JD, Schmidt D, Grant RM, Halvorsen RA, Schambelan M, McCune JM: Increased thymic mass and circulating naive CD4 T cells in HIV-1-infected adults treated with growth hormone. AIDS. 2002, 16: 1103-1111. 10.1097/00002030-200205240-00003.
Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, Boué F, Molina JM, Rouzioux C, Avettand-Fénoêl V, Croughs T, Beq S, Thiébaut R, Chêne G, Morre M, Delfraissy JF: Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009, 119: 997-1007.
Seder RA, Grabstein KH, Berzofsky JA, McDyer JF: Cytokine interactions in human immunodeficiency virus-infected individuals: roles of interleukin (IL)-2, IL-12, and IL-15. J Exp Med. 1995, 182: 1067-1077. 10.1084/jem.182.4.1067.
Mueller YM, Do DH, Altork SR, Artlett CM, Gracely EJ, Katsetos CD, Legido A, Villinger F, Altman JD, Brown CR, Lewis MG, Katsikis PD: IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J Immunol. 2008, 180: 350-360. 10.4049/jimmunol.180.1.350.
Pallikkuth S, Rogers K, Villinger F, Dosterii M, Vaccari M, Franchini G, Pahwa R, Pahwa S: Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine. 2011, 29: 9229-9238. 10.1016/j.vaccine.2011.09.118.
Vignali DA, Kuchroo VK: IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012, 13: 722-728. 10.1038/ni.2366.
Hunter CA: New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005, 5: 521-531. 10.1038/nri1648.
Chen Q, Swaminathan S, Yang D, Dai L, Sui H, Yang J, Hornung RL, Wang Y, Huang da W, Hu X, Lempicki RA, Imamichi T: Interleukin-27 is a potent inhibitor of cis HIV-1 replication in monocyte-derived dendritic cells via a type I interferon-independent pathway. PLoS One. 2013, 8: e59194-10.1371/journal.pone.0059194.
Dai L, Lidie KB, Chen Q, Adelsberger JW, Zheng X, Huang D, Yang J, Lempicki RA, Rehman T, Dewar RL, Wang Y, Hornung RL, Canizales KA, Lockett SJ, Lane HC, Imamichi T: IL-27 inhibits HIV-1 infection in human macrophages by down-regulating host factor SPTBN1 during monocyte to macrophage differentiation. J Exp Med. 2013, 210: 517-534. 10.1084/jem.20120572.
Park K: Nanotechnology: what it can do for drug delivery. J Control Release. 2007, 120: 1-3. 10.1016/j.jconrel.2007.05.003.
Langer R: Drug delivery. Drugs on target. Science. 2001, 293: 58-59. 10.1126/science.1063273.
Wan L, Pooyan S, Hu P, Leibowitz MJ, Stein S, Sinko PJ: Peritoneal macrophage uptake, pharmacokinetics and biodistribution of macrophage-targeted PEG-fMLF (N-formyl-methionyl-leucyl-phenylalanine) nanocarriers for improving HIV drug delivery. Pharm Res. 2007, 24: 2110-2119. 10.1007/s11095-007-9402-5.
Vandamme AM, Van VK, De CE: Anti-human immunodeficiency virus drug combination strategies. Antivir Chem Chemother. 1998, 9: 187-203.
Clifford DB: HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med. 2008, 16: 94-98.
Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CHARTER Group: HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010, 75: 2087-2096. 10.1212/WNL.0b013e318200d727.
Zink MC, Brice AK, Kelly KM, Queen SE, Gama L, Li M, Adams RJ, Bartizal C, Varrone J, Rabi SA, Graham DR, Tarwater PM, Mankowski JL, Clements JE: Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Infect Dis. 2010, 202: 161-170. 10.1086/653213.
Kim HJ, Martemyanov KA, Thayer SA: Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death. J Neurosci. 2008, 28: 12604-12613. 10.1523/JNEUROSCI.2958-08.2008.
Lu SM, Tremblay ME, King IL, Qi J, Reynolds HM, Marker DF, Varrone JJ, Majewska AK, Dewhurst S, Gelbard HA: HIV-1 Tat-induced microgliosis and synaptic damage via interactions between peripheral and central myeloid cells. PLoS One. 2011, 6: e23915-10.1371/journal.pone.0023915.
Bagashev A, Sawaya BE: Roles and functions of HIV-1 Tat protein in the CNS: an overview. Virol J. 2013, 10: 358-10.1186/1743-422X-10-358.
Rodriguez-Franco EJ, Cantres-Rosario YM, Plaud-Valentin M, Romeu R, Rodriguez Y, Skolasky R, Meléndez V, Cadilla CL, Melendez LM: Dysregulation of macrophage-secreted cathepsin B contributes to HIV-1-linked neuronal apoptosis. PLoS One. 2012, 7: e36571-10.1371/journal.pone.0036571.
Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF: In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995, 1: 1284-1290. 10.1038/nm1295-1284.
Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF: Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997, 387: 183-188. 10.1038/387183a0.
Eisele E, Siliciano RF: Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012, 37: 377-388. 10.1016/j.immuni.2012.08.010.
Zhu T, Muthui D, Holte S, Nickle D, Feng F, Brodie S, Hwangbo Y, Mullins JI, Corey L: Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol. 2002, 76: 707-716. 10.1128/JVI.76.2.707-716.2002.
Alexaki A, Wigdahl B: HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. PLoS Pathog. 2008, 4: e1000215-10.1371/journal.ppat.1000215.
Redel L, Le Douce V, Cherrier T, Marban C, Janossy A, Aunis D, Van Lint C, Rohr O, Schwartz C: HIV-1 regulation of latency in the monocyte-macrophage lineage and in CD4+ T lymphocytes. J Leukoc Biol. 2010, 87: 575-588.
McElrath MJ, Steinman RM, Cohn ZA: Latent HIV-1 infection in enriched populations of blood monocytes and T cells from seropositive patients. J Clin Invest. 1991, 87: 27-30. 10.1172/JCI114981.
Caselli E, Galvan M, Cassai E, Caruso A, Sighinolfi L, Di LD: Human herpesvirus 8 enhances human immunodeficiency virus replication in acutely infected cells and induces reactivation in latently infected cells. Blood. 2005, 106: 2790-2797. 10.1182/blood-2005-04-1390.
Jones J, Kosloff BR, Benveniste EN, Shaw GM, Kutsch O: Amphotericin-B-mediated reactivation of latent HIV-1 infection. Virology. 2005, 331: 106-116. 10.1016/j.virol.2004.10.013.
Gonzalez OA, Li M, Ebersole JL, Huang CB: HIV-1 reactivation induced by the periodontal pathogens Fusobacterium nucleatum and Porphyromonas gingivalis involves Toll-like receptor 2 [corrected] and 9 activation in monocytes/macrophages. Clin Vaccine Immunol. 2010, 17: 1417-1427. 10.1128/CVI.00009-10.
Huang CB, Alimova YV, Strange S, Ebersole JL: Polybacterial challenge enhances HIV reactivation in latently infected macrophages and dendritic cells. Immunology. 2011, 132: 401-409. 10.1111/j.1365-2567.2010.03375.x.
Le Douce V, Herbein G, Rohr O, Schwartz C: Molecular mechanisms of HIV-1 persistence in the monocyte-macrophage lineage. Retrovirology. 2010, 7: 32-10.1186/1742-4690-7-32.
Marban C, Redel L, Suzanne S, Van Lint C, Lecestre D, Chasserot-Golaz S, Leid M, Aunis D, Schaeffer E, Rohr O: COUP-TF interacting protein 2 represses the initial phase of HIV-1 gene transcription in human microglial cells. Nucleic Acids Res. 2005, 33: 2318-2331. 10.1093/nar/gki529.
Kumar A, Abbas W, Herbein G: TNF and TNF receptor superfamily members in HIV infection: new cellular targets for therapy?. Mediators Inflamm. 2013, 2013: 484378-
Lehrman G, Hogue IB, Palmer S, Jennings C, Spina CA, Wiegand A, Landay AL, Coombs RW, Richman DD, Mellors JW, Coffin JM, Bosch RJ, Margolis DM: Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005, 366: 549-555. 10.1016/S0140-6736(05)67098-5.
Siliciano JD, Lai J, Callender M, Pitt E, Zhang H, Margolick JB, Gallant JE, Cofrancesco J Jr, Moore RD, Gange SJ, Siliciano RF: Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J Infect Dis. 2007, 195: 833-836. 10.1086/511823.
Sagot-Lerolle N, Lamine A, Chaix ML, Boufassa F, Aboulker JP, Costagliola D, Goujard C, Pallier C, Delfraissy JF, Lambotte O, ANRS EP39 study: Prolonged valproic acid treatment does not reduce the size of latent HIV reservoir. AIDS. 2008, 22: 1125-1129. 10.1097/QAD.0b013e3282fd6ddc.
Savarino A, Mai A, Norelli S, El DS, Valente S, Rotili D, Altucci L, Palamara AT, Garaci E: “Shock and kill” effects of class I-selective histone deacetylase inhibitors in combination with the glutathione synthesis inhibitor buthionine sulfoximine in cell line models for HIV-1 quiescence. Retrovirology. 2009, 6: 52-10.1186/1742-4690-6-52.
Matalon S, Palmer BE, Nold MF, Furlan A, Kassu A, Fossati G, Mascagni P, Dinarello CA: The histone deacetylase inhibitor ITF2357 decreases surface CXCR4 and CCR5 expression on CD4(+) T-cells and monocytes and is superior to valproic acid for latent HIV-1 expression in vitro. J Acquir Immune Defic Syndr. 2010, 54: 1-9.
Furlan A, Monzani V, Reznikov LL, Leoni F, Fossati G, Modena D, Mascagni P, Dinarello CA: Pharmacokinetics, safety and inducible cytokine responses during a phase 1 trial of the oral histone deacetylase inhibitor ITF2357 (givinostat). Mol Med. 2011, 17: 353-362.
Dallas S, Miller DS, Bendayan R: Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev. 2006, 58: 140-161. 10.1124/pr.58.2.3.
Shikuma CM, Nakamoto B, Shiramizu B, Liang CY, DeGruttola V, Bennett K, Paul R, Kallianpur K, Chow D, Gavegnano C, Hurwitz SJ, Schinazi RF, Valcour VG: Antiretroviral monocyte efficacy score linked to cognitive impairment in HIV. Antivir Ther. 2012, 17: 1233-1242. 10.3851/IMP2411.
Acknowledgements
This work was supported by grants from the University of Franche-Comté (UFC) and the Région Franche-Comté (RECH-FON12-000013) to G.H. A.K. is a recipient of a postdoctoral fellowship of the Region Franche-Comté.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
GH is a member of the editorial board of Molecular and Cellular Therapies. No other competing interests are declared.
Authors’ contributions
AK and GH wrote the manuscript. Both authors read and approved this manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kumar, A., Herbein, G. The macrophage: a therapeutic target in HIV-1 infection. Mol and Cell Ther 2, 10 (2014). https://doi.org/10.1186/2052-8426-2-10
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2052-8426-2-10