Abstract
Background and the purpose of the study
Modified androsterone derivatives are class of steroidal compounds with potential anticancer properties. Various steroidal derivatives containing substitution at position 16 have shown diversified pharmacological activities. In the present study, a new series of cytotoxic 16-(substituted benzylidene) derivatives of dehydroepiandrosterone (DHEA) were synthesized and evaluated against three different cancer cell lines.
Methods
The cytotoxic 16-(substituted benzylidene) derivatives of DHEA were synthesized via aldol condensation of DHEA with corresponding benzaldehyde derivatives. The cytotoxic activity of synthesized derivatives was evaluated against three different cancer cells including KB, T47D and SK-N-MC cell lines by MTT reduction colorimetric assay.
Results
The results indicated that 16-(substituted benzylidene) derivatives of DHEA could be served as a potent anti-cancer agent. The 3-cholro benzylidene derivatives of DHEA was the most potent synthesized derivative especially against KB and T47D cell lines (IC50 values were 0.6 and 1.7 μM; respectively).
Conclusion
The cytotoxic potential of novel benzylidene derivatives of DHEA is mainly attributed to the position and nature of the substituted group on the benzylidene pendant.
Similar content being viewed by others
Introduction
Steroidal derivatives are important class of synthetic and naturally occurring compounds, which have exhibited different biological properties [1–3] and attracted profound attention for development of potent pharmacological agents for treatments of various diseases [4] including: cardiovascular disease [5], adrenal insufficiencies [6], autoimmune disorders [7], fungal and microbial infections [8, 9]. Furthermore, different steroidal derivatives have been considered as potent anti-cancer agents for the treatment of leukemia [4], breast cancer [10–12], prostate cancer [13] and brain tumors [14].
Several natural and modified steroidal derivatives have been previously described in the literatures as potent cytotoxic agents [15–17]. In this regard, different derivatives of androsterone (3α-hydroxy-5α-androstan-17-one) have been excessively studied as potent anti-cancer agents (Figure 1) [18, 19]. Recently, the significant cytotoxic and aromatase inhibitory potential of a large number of androsterone derivatives containing substitution at position 16 have been reported [12, 20, 21]. Bansal et. al demonstrated the effectiveness of different 16E-arylidenosteroids as potential anticancer and anti-aromatase scaffold against estrogen-dependent breast cancer and different human tumor cell lines [12, 20]. The cytotoxic mechanistic study of α,β-unsaturated carbonyl derivatives revealed that compounds containing described functional group can cause alteration and mis-folding of proteins through the formation of adducts with reactive thiol groups of proteins [22]. For this reason, the α-β unsaturated androstrone derivatives containing exocyclic double bond at C16 position could be served as potent chemotherapeutic agents. Dehydroepiandrosterone (DHEA), also known as androstenolone (3β-hydroxyandrost-5-en-17-one) is an androsterone derivative and important endogenous steroid hormone which plays an important role as intermediate for biosynthesis of androgens and estrogen hormones [23]. Apart from its different biological potential, DHEA demonstrated antiproliferative and antiapoptotic effects on different cancer cell lines [24–26].
In the course of our ongoing study for the synthesis and biological evaluation of potential anticancer agents [27–34], herein, we investigate the synthesis and cytotoxic activity evaluation of a new series of 16-(substituted benzylidene) derivatives of DHEA taking into account the structural necessities for cytotoxic activity of these derivatives. The aim of this study was to investigate the structural requirements affecting the cytotoxic potential of modified steroidal compounds.
Material and methods
Chemistry
All starting materials, reagents, and solvents were prepared from Merck AG (Germany). Thin layer chromatography (TLC) using various solvents of different polarities was applied for determination of the purity of the synthesized compounds. Melting points were determined on a Kofler hot stage apparatus (Vienna, Austria) and are uncorrected. 1H-NMR spectra were recorded using a Bruker 400 spectrometer (Bruker, Rheinstatten, Germany), and chemical shifts are expressed as δ (ppm) with tetramethylsilane (TMS) as internal standard. The IR spectra were recorded using a Shimadzu 470 (Shimadzu, Tokyo, Japan) spectrophotometer (potassium bromide disks).The mass spectra were recorded on a Finnigan TSQ-70 spectrometer (Finnigan, USA) at 70 eV.
General procedure for the preparation of the (E)-16-(substituted benzylidene) dehydroepiandrosterone derivatives 1a-m using aldol Condensation
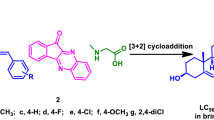
The appropriate aldehyde was added to a mixture of DHEA (1.0 g, 3.47 mmol) and NaOH (1.75 g) in methanol (20 ml). The reaction mixture was stirred for 1 h at room temperature. The completion of reaction was confirmed using analytical thin layer chromatography. After completion, the reaction mixture was poured into ice-water. The final precipitate was filtered; washed with cold water, dried under reduced pressure and crystallized in methanol.
(E)-16-(2-Chlorobenzylidene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-3-hydroxy-10,13-dimethyl-2H-cyclopenta[a]phenanthren-17(14H)-one (1a)
Yield: 23%; mp=213-214◦°C; IR (KBr, νmax, cm-1): 3475(OH), 1725(C=O).1HNMR (400 MHz, CDCl3): 1.00(s, 3H, CH3), 1.07 (s, 3H, CH3), 3.52-3.63(m, 1H, CH-OH), 5.40(s, 1H, Hvinyl), 7.28-7.32(m, 2H, Hphenyl), 7.42-7.46(m, 1H, Hphenyl), 7.52-7.56(m,1H, Hphenyl). MS (EI) m/z (%): 412 (M++2, 31), 410(M+, 100).
(E)-16-(3-Chlorobenzylidene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-3-hydroxy-10,13-dimethyl-2H-cyclopenta[a]phenanthren-17(14H)-one (1b)
Yield: 30%; mp= 199-202◦°C; IR (KBr, νmax, cm-1): 3219 (OH), 1708 (C=O).1HNMR (400 MHz, CDCl3): 0.99(s, 3H, CH3), 1.07(s, 3H, CH3), 3.48-3.60(m, 1H, CH-OH), 5.41(s, 1H, Hvinyl), 7.33-7.43(m, 4H, Hphenyl). MS (EI) m/z (%): 412 (M++2, 5), 410 (M+, 15).
(E)-16-(4-Chlorobenzylidene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-3-hydroxy-10,13-dimethyl-2H-cyclopenta[a]phenanthren-17(14H)-one (1c)
Yield: 27%; mp=229-231◦°C; IR (KBr, νmax, cm-1): 3416(OH), 1710(C=O). 1HNMR (400 MHz, CDCl3): 0.98(s, 3H, CH3), 1.07(s, 3H, CH3), 3.53-3.54(m, 1H, CH-OH), 5.40(s, 1H, Hvinyl), 7.39(dd, 1H, Hphenyl, J= 8.5Hz ),7.46(dd, 1H, Hphenyl, J= 8.5Hz ). MS (EI) m/z (%): 412 (M++2, 10), 410 (M+, 28), 378(18), 351(4), 300(19), 268(10), 214(22), 150(100), 91(87), 79(100).
(E)-16-(2,4-Dichlorobenzylidene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-3-hydroxy-10,13-dimethyl-2H-cyclopenta[a]phenanthren-17(14H)-one (1d)
Yield: 35%; mp=203-205◦°C; IR (KBr, νmax, cm-1): 3472 (OH), 2929(CH aliphatic), 1710(C=O).1H-NMR (DMSO-d6): 1HNMR (400 MHz, CDCl3): 1.03(s, 3H, CH3), 1.11(s, 3H, CH3), 3.55-3.56(m, 1H, CH-OH), 5.40(s, 1H, Hvinyl), 7.48-7.49(m, 2H, Hphenyl), 7.48(s, 1H, Hphenyl), 7.70-7.72(m, 1H, Hphenyl). MS (EI) m/z (%): 445(M+,10), 410(100), 343(18), 297(10), 213(11), 186(18), 105(9), 57(18).
(E)-16-(4-Fluorobenzylidene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-3-hydroxy-10,13-dimethyl-2H-cyclopenta[a]phenanthren-17(14H)-one (1e)
Yield: 46%; mp=234-236◦°C; IR (KBr, νmax, cm-1): 3426(OH), 1716(C=O). 1HNMR (400 MHz, CDCl3): 0.98(s, 3H, CH3), 1.07(s, 3H, CH3), 3.48-3.58(m, 1H, CH-OH), 5.40(s, 1H, Hvinyl), 7.10(t, 1H, Hphenyl, J= 8.5 Hz), 7.52(t, 1H, Hphenyl, J= 8.5Hz). MS (EI) m/z (%): 395(M+, 38), 377(10), 284(10), 232(32), 203(39), 145(25), 134(100), 109(25), 82(12).
(E)-16-(3-Bromobenzylidene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-3-hydroxy-10,13-dimethyl-2H-cyclopenta[a]phenanthren-17(14H)-one (1f)
Yield: 28%; mp=222-234◦°C, IR (KBr, νmax, cm-1): 3471(OH), 1709 (C=O). 1HNMR (400 MHz, CDCl3):0.95(s, 3H, CH3), 1.04(s, 3H, CH3), 3.48-3.60( m, 1H, CH-OH), 5.40(s, 1H, HVinyl), 7.29(t, 1H, Hphenyl, J= 6.4Hz), 7.35(s, 1H, Hphenyl), 7.46(d, 1H, Hphenyl, J= 6.4Hz), 7.49(d, 1H, Hphenyl, J= 6.4Hz). MS (EI) m/z (%): 456(M+, 26), 454(M+, 26), 436(46), 424(32), 343(26), 315(18), 263(32), 213(38).
(E)-16-(5-Bromo-2-hydroxybenzylidene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-3-hydroxy-10,13-dimethyl-2H-cyclopenta[a]phenanthren-17(14H)-one (1g)
Yield: 46%; mp=208-210◦°C; IR (KBr, νmax, cm-1): 3464(OH), 1709(C=O). 1HNMR (400 MHz, CDCl3): 1.00(s, 3H, CH3), 1.07 (s, 3H, CH3), 3.42-3.60(m, 1H, CH-OH),5.40(s, 1H, Hvinyl), 6.83(dd, 1H, Hphenyl, J= 8.4Hz), 7.32(dd, 1H, Hphenyl, J= 8.4Hz), 7.52(d, 1H, Hphenyl, J= 8.4Hz). MS (EI) m/z (%): 472 (M++2, 95), 470(M+, 95).
(E)-16-(2-(Trifluoromethyl)benzylidene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-3-hydroxy-10,13-dimethyl-2H-cyclopenta[a]phenanthren-17(14H)-one (1h)
Yield: 34%; mp = 208-210◦°C; IR (KBr, νmax, cm-1): 3456(OH), 1724(C=O).1HNMR (400 MHz, CDCl3): 1.00(s, 3H, CH3), 1.06 (s, 3H, CH3), 3.48-3.60(m, 1H, CH-OH), 5.38(s, 1H, Hvinyl), 7.42-48(m, 1H, Hphenyl) 7.56-7.60 (m, 2H, Hphenyl), 7.70-7.75(m, 2H, Hphenyl-HVinyl ). MS (EI) m/z (%): 445 (M++1, 14), 444(M+, 100).
(E)-16-(4-(Trifluoromethyl)benzylidene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-3-hydroxy-10,13-dimethyl-2H-cyclopenta[a]phenanthren-17(14H)-one (1i)
Yield: 27%; mp=244-246◦°C; IR (KBr, νmax, cm-1): 3215(OH), 1708(C=O). 1HNMR (400 MHz, CDCl3): 0.99(s, 3H, CH3), 1.08(s, 3H, CH3), 3.48-3.60(m, 1H, CH-OH), 7.40(s, 1H, Hvinyl), 7.38-7.46(m, 2H, Hphenyl), 7.64(dd, 2H, Hphenyl, J=8.8Hz). MS (EI) m/z (%): 445 (M++1, 21), 444(M+, 100).
(E)-16-(4-Methylbenzylidene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-3-hydroxy-10,13-dimethyl-2H-cyclopenta[a]phenanthren-17(14H)-one (1j)
Yield: 78%; mp=238-240◦°C; IR (KBr, νmax, cm-1): 3421(OH), 1716(C=O). 1HNMR (400 MHz, CDCl3): 0.98(s, 3H, CH3), 1.07(s, 3H, CH3), 1.57(s, 3H, CH3), 3.45-3.50(m, 1H, CH-OH), 5.42(s, 1H, Hvinyl), 7.25(dd, 1H, HPhenyl, J=8.4Hz), 7.44(dd, 1H, HPhenyl, J=8.4 Hz). MS (EI) m/z (%): 391 (M++1, 9), 390 (M+, 44), 376(4), 131(100).
(E)-16-(4-Methoxybenzylidene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-3-hydroxy-10,13-dimethyl-2H-cyclopenta[a]phenanthren-17(14H)-one(1k)
Yield: 25%; mp =225-227◦°C; IR (KBr, νmax, cm-1): 3454(OH), 1712(C=O). 1HNMR (400 MHz, CDCl3): 0.97(s, 3H, CH3), 1.07(s, 3H, CH3), 3.85(s, 3H, OCH3), 3.50-3.60(m, 1H, CH-OH), 5.40(s, 1H, Hvinyl), 6.94(dd, 1H, Hphenyl, J=8.4Hz), 7.40(s, 1H, Hvinyl), 7.51(dd, 1H, Hphenyl, J=8.4Hz). MS (EI) m/z (%): 407(M++1, 7), 406(M+, 29), 408(100).
(E)-16-(2,3,4-Trimethoxybenzylidene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-3-hydroxy-10,13-dimethyl-2H-cyclopenta[a]phenanthren-17(14H)-one (1l)
Yield: 20%; mp=199-201◦°C; IR (KBr, νmax, cm-1): 3429(OH), 1717(C=O).1HNMR (400 MHz, CDCl3): 0.98(s, 3H, CH3), 1.07(s, 3H, CH3), 3.90(s, 9H, 3OCH3), 5.39(s, 1H, Hvinyl), 6.72(d, 1H, HPhenyl, J=8.4Hz), 7.27(d, 1H, HPhenyl,J=8.4Hz). MS (EI) m/z (%):467(M++1, 28), 466(M+, 100).
(E)-16-(4-(Dimethylamino)benzylidene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-3-hydroxy-10,13-dimethyl-2H-cyclopenta[a]phenanthren-17(14H)-one (1m)
Yield: 35%; mp=216-218◦°C; IR (KBr, νmax, cm-1): 3521(OH), 1721(C=O). 1HNMR (400 MHz, CDCl3): 0.96(s, 3H, CH3), 1.07(s, 3H, CH3), 3.03(s, 6H, 2CH3), 3.42-3.60(m, 1H, CH-OH), 5.42(s, 1H, Hvinyl), 6.71(d, 1H, Hphenyl, J= 8.3Hz), 7.47(d, 1H, Hphenyl, J= 8.3Hz). MS (EI) m/z (%): 420(M++1, 32), 419(M+, 10).
Biological assay
Cell lines and cell culture
The synthesized compounds were tested against three different human cancer cell lines including KB (human nasopharyngeal epidermoid carcinoma), T47D (human breast cancer) and SK-N-MC (human neuroblastoma) cells. The cell lines were purchased from the National Cell Bank of Iran (NCBI). The cells were grown in RPMI- 1640 medium (Gibco BRL) supplemented with 10% heat inactivated fetal calf serum (Gibco BRL), 100 μg/mL streptomycin, and 100 U/mL penicillin, in a humidified air atmosphere at 37°C with 5% CO2.
In vitro cytotoxicity assay
The in vitro cytotoxic activity of each synthesized derivatives 1a-m was investigated using MTT colorimetric assay [35]. Briefly, each cell line in log-phase of growth was harvested by trypsinization followed by resuspension in complete growth medium to give a total cell count of 5×104 cells/ml. The resulted cell suspension was seeded into the wells of 96-well plates (Nunc, Denmark). The plates were incubated overnight in a humidified air atmosphere at 37°C with 5% CO2. After the incubation period, 5 μL of the media containing various concentrations of the compounds was added per well in triplicate followed by further incubation for 24 h. The final maximum concentration of DMSO was 0.1%. Etoposide was used as positive control for cytotoxic activity, while three different wells containing evaluated cancer cells cultured in 200 μL of complete medium were used as negative controls of cell viability. After incubation, the medium was discarded and 200 μl phenol red-free RPMI containing MTT (final concentration 1 mg/ml), was added to each well. The test plate was incubated for 4h. The culture medium was then replaced with 100 μL of DMSO and the absorbance of each well was measured by using a micro plate reader (Gen5, Power wave xs2, BioTek, America) at 492 nm. Each set of experiments was independently performed three times. The concentration causing 50% cell growth inhibition (IC50) compared with the control was calculated using concentration-response curves by regression analysis.
Results and discussion
The benzylidene-substituted DHEA derivatives 1a-m were synthesized through the aldol condensation [36] of DHEA with corresponding benzaldehyde derivatives (Figure 2).
The in vitro cytotoxic activity of synthesized compounds 1a-m was investigated against three different cancer cell lines including KB, T47D and SK-N-M cells. The percentage of growth inhibition was assessed using MTT reduction assay versus controls not treated with test compounds. The 50% growth inhibitory concentration (IC50) for each compound was determined and presented in Table 1. The data for etoposide was also included.
The results of cytotoxic data indicate that most of synthesized compounds showed moderate to strong cytotoxic potential in all three cell lines. Based on the cytotoxic data, the following structure-activity relationship may be developed:
-
Introduction of different substitutes such as chlorine, trifluoromethyl, methoxy and methyl groups into the ortho or meta position of benzylidene moiety, resulted in enhanced cytotoxic potential of benzylidene derivatives of DHEA.
-
The compounds containing chlorine, nitro and fluorine substitutes at para position of benzylidene pendant, were almost inactive against all three evaluated cell lines (IC50>100 μM). Whereas, substitution of methoxy, methyl and trifluoromethyl groups into the para position (compounds 1i-j), resulted in enhanced cytotoxic potential of corresponding derivatives, e.g. the corresponding IC50 values of para-methyl benzylidene derivative 1j in KB, T47D and SK-N-MC cell lines were 1.7, 7.6 and 1.0 μM, respectively.
-
The 3-chloro benzylidene derivatives of DHEA, compound 1b, was the most potent synthesized derivative especially against KB and T47D cell lines (IC50 values were 0.6 and 1.7 μM; respectively) which were comparable with etoposide (IC50= 2.8 and 1.2 μM; respectively).
Based on the above finding it might be deduced that 16-(substituted benzylidene) derivatives of DHEA could be served as a potent anti-cancer agents. The cytotoxic potential of described compounds is mainly attributed to the position and nature of the substituted group on the benzylidene pendant. The ortho or meta positions of the benzylidene group could well accommodate different substitute in order to afford potent cytotoxic derivatives of this types.
Conclusion
A new series of cytotoxic 16-(substituted benzylidene) derivatives of DHEA were synthesized and evaluated against three different cancer cells including KB, T47D and SK-N-MC cell lines by MTT reduction colorimetric assay. The cytotoxic potential of these novel benzylidene derivatives of DHEA is mainly attributed to the position and nature of the substituted group on the benzylidene pendant.
References
Mensah-Nyagan AG, Meyer L, Schaeffer V, Kibaly C, Patte-Mensah C: Evidence for a key role of steroids in the modulation of pain. Psychoneuroendocrino. 2009, 34: 169-177.
Ibrahim-Ouali M: Recent advances in oxasteroids chemistry. Steroids. 2007, 72: 475-508. 10.1016/j.steroids.2007.03.004.
Bhatti HN, Khera RA: Biological transformations of steroidal compounds: A review. Steroids. 2012, 77: 1267-1290. 10.1016/j.steroids.2012.07.018.
Bansal R, Guleria S, Thota S, Bodhankar SL, Patwardhan MR, Zimmer C: Design, synthesis and evaluation of novel 16-imidazolyl substituted steroidal derivatives possessing potent diversified pharmacological properties. Steroids. 2012, 77: 621-629. 10.1016/j.steroids.2012.02.005.
Dubey RK, Oparil S, Imthurn B, Jackson EK: Sex hormones and hypertension. Cardiovasc Res. 2002, 53: 688-708. 10.1016/S0008-6363(01)00527-2.
Holst JP, Soldin SJ, Tractenberg RE, Guo T, Kundra P, Verbalis JG: Use of steroid profiles in determining the cause of adrenal insufficiency. Steroids. 2007, 72: 71-84. 10.1016/j.steroids.2006.11.001.
Auci DL, Reading CL, Frincke JM: 7-Hydroxy androstene steroids and a novel synthetic analogue with reduced side effects as a potential agent to treat autoimmune diseases. Autoimmun Rev. 2009, 8: 369-372. 10.1016/j.autrev.2008.11.011.
Jursic BS, Upadhyay SK, Creech CC, Neumann DM: Novel and efficient synthesis and antifungal evaluation of 2,3-functionalized cholestane and androstane derivatives. Bioorg Med Chem Lett. 2010, 20: 7372-7375. 10.1016/j.bmcl.2010.10.044.
Banday AH, Iqbal Zargar M, Ganaie BA: Synthesis and antimicrobial studies of chalconyl pregnenolones. Steroids. 2011, 76: 1358-1362. 10.1016/j.steroids.2011.07.001.
Billich A, Nussbaumer P, Lehr P: Stimulation of MCF-7 breast cancer cell proliferation by estrone sulfate and dehydroepiandrosterone sulfate: inhibition by novel non-steroidal steroid sulfatase inhibitors. J Steroid Biochem. 2000, 73: 225-235. 10.1016/S0960-0760(00)00077-7.
Saha P, Fortin S, Leblanc V, Parent S, Asselin E, Berube G: Design, synthesis, cytocidal activity and estrogen receptor alpha affinity of doxorubicin conjugates at 16alpha-position of estrogen for site-specific treatment of estrogen receptor positive breast cancer. Steroids. 2012, 77: 1113-1122. 10.1016/j.steroids.2012.06.004.
Bansal R, Guleria S, Thota S, Hartmann RW, Zimmer C: Synthesis and biological evaluation of 16E-arylidenosteroids as cytotoxic and anti-aromatase agents. Chem Pharma Bull. 2011, 59: 327-331. 10.1248/cpb.59.327.
Gauthier S, Martel C, Labrie F: Steroid derivatives as pure antagonists of the androgen receptor. J Steroid Biochem. 2012, 132: 93-104. 10.1016/j.jsbmb.2012.02.006.
Sheridan PJ, Blum K, Trachtenberg MC: Steroid receptors and disease: cancer, autoimmune, bone, and circulatory disorders 1988, 289-564. Marcel Dekker Inc.
Li C, Qiu W, Yang Z, Luo J, Yang F, Liu M: Stereoselective synthesis of some methyl-substituted steroid hormones and their in vitro cytotoxic activity against human gastric cancer cell line MGC-803. Steroids. 2010, 75: 859-869. 10.1016/j.steroids.2010.05.008.
Li Y, Huang J, Liu J, Yan P, Liu H, Sun Q: Synthesis and cytotoxicity of 17E-(2-aryl-2-oxo-1-ethylidene)-5alpha-androstane derivatives. Steroids. 2011, 76: 1615-1620. 10.1016/j.steroids.2011.10.003.
Duh CY, Lo IW, Wang SK, Dai CF: New cytotoxic steroids from the soft coral Clavularia viridis. Steroids. 2007, 72: 573-579. 10.1016/j.steroids.2007.03.010.
Banday AH, Singh S, Alam MS, Reddy DM, Gupta BD, Sampath Kumar HM: Synthesis of novel steroidal D-ring substituted isoxazoline derivatives of 17-oxoandrostanes. Steroids. 2008, 73: 370-374. 10.1016/j.steroids.2007.10.014.
Krstic NM, Bjelakovic MS, Pavlovic VD, Robeyns K, Juranic ZD, Matic I: New androst-4-en-17-spiro-1,3,2-oxathiaphospholanes. Synthesis, assignment of absolute configuration and in vitro cytotoxic and antimicrobial activities. Steroids. 2012, 77: 558-565. 10.1016/j.steroids.2012.01.021.
Bansal R, Thota S, Karkra N, Minu M, Zimmer C, Hartmann RW: Synthesis and aromatase inhibitory activity of some new 16E-arylidenosteroids. Bioorg Chem. 2012, 45: 36-40.
Chattopadhaya R, Jindal DP, Minu M, Gupta R: Synthesis and cytotoxic studies of hydroximino derivatives of some 16E-arylidenosteroids. Arzneimittel-Forsch. 2004, 54: 551-556.
Bradshaw TD, Matthews CS, Cookson J, Chew EH, Shah M, Bailey K: Elucidation of thioredoxin as a molecular target for antitumor quinols. Cancer Res. 2005, 65: 3911-3919. 10.1158/0008-5472.CAN-04-4141.
Mo Q, Lu SF, Simon NG: Dehydroepiandrosterone and its metabolites: differential effects on androgen receptor trafficking and transcriptional activity. J Steroid Biochem. 2006, 99: 50-58. 10.1016/j.jsbmb.2005.11.011.
Yang NC, Jeng KC, Ho WM, Hu ML: ATP depletion is an important factor in DHEA-induced growth inhibition and apoptosis in BV-2 cells. Life Sci. 2002, 70: 1979-1988. 10.1016/S0024-3205(01)01542-9.
Loria RM: Immune up-regulation and tumor apoptosis by androstene steroids. Steroids. 2002, 67: 953-966. 10.1016/S0039-128X(02)00043-0.
Tworoger SS, Sluss P, Hankinson SE: Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res. 2006, 66: 2476-2482. 10.1158/0008-5472.CAN-05-3369.
Safavi M, Esmati N, Ardestani SK, Emami S, Ajdari S, Davoodi J, Shafiee A, Foroumadi A: Halogenated flavanones as potential apoptosis-inducing agents: synthesis and biological activity evaluation. Eur J Med Chem. 2012, 58: 573-580.
Nakhjiri M, Safavi M, Alipour E, Emami S, Atash AF, Jafari-Zavareh M: Asymmetrical 2, 6-bis (benzylidene) cyclohexanones: Synthesis, cytotoxic activity and QSAR study. Eur J Med Chem. 2012, 50: 113-123.
Aryapour H, Mahdavi M, Mohebbi SR, Zali MR, Foroumadi A: Anti-proliferative and apoptotic effects of the derivatives from 4-aryl-4H-chromene family on human leukemia K562 cells. Arch Pharm Res. 2012, 35: 1573-1582. 10.1007/s12272-012-0908-y.
Rafinejad A, Fallah-Tafti A, Tiwari R, Shirazi AN, Mandal D, Shafiee A, Parang K, Foroumadi A, Akbarzadeh T: 4-Aryl-4H-naphthopyrans derivatives: One-pot synthesis, evaluation of Src kinase inhibitory and anti-proliferative activities. DARU J Pharmaceut Sci. 2012, 20: 100-10.1186/2008-2231-20-100.
Firoozpour L, Edraki N, Nakhjiri M, Emami S, Safavi M, Ardestani SK: Cytotoxic activity evaluation and QSAR study of chromene-based chalcones. Arch Pharm Res. 2012, 35: 2117-2125. 10.1007/s12272-012-1208-2.
Bazl R, Ganjali MR, Saboury AA, Foroumadi A, Nourozi P, Amanlou M: A new strategy based on pharmacophore-based virtual screening in adenosine deaminase inhibitors detection and in-vitro study. DARU J Pharmaceut Sci. 2012, 20: 64-69. 10.1186/2008-2231-20-64.
Noushini S, Emami S, Safavi M, Kabudanian Ardestani S, Gohari AR, Shafiee A, Foroumadi A: Synthesis and cytotoxic properties of novel (E)-3-benzylidene-7-methoxychroman-4-one derivatives. DARU J Pharmaceut Sci. 2013, 21: 31-10.1186/2008-2231-21-31.
Aryapour H, Riazi GH, Ahmadian S, Foroumadi A, Mahdavi M, Emami S: Induction of apoptosis through tubulin inhibition in human cancer cells by new chromene-based chalcones. Pharm Biol. 2012, 50: 1551-1560. 10.3109/13880209.2012.695799.
Mosmann T: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983, 65: 55-63. 10.1016/0022-1759(83)90303-4.
Nadri H, Pirali-Hamedani M, Moradi A, Sakhteman A, Vahidi A, Sheibani V, Asadipour A, Hosseinzadeh N, Abdollahi M, Shafiee A, Foroumadi A: 5,6-Dimethoxybenzofuran-3-one derivatives: a novel series of dual Acetylcholinesterase/Butyrylcholinesterase inhibitors bearing benzyl pyridinium moiety. DARU J Pharmaceut Sci. 2013, 21: 15-23. 10.1186/2008-2231-21-15.
Acknowledgement
This work has been supported by grants from Research Council of Tehran University of Medical Sciences and Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MV: Design and synthesis of target compounds. HY: Synthesis of some intermediates and target compounds, KD: performing the biological tests, HS: Synthesis of some target compounds, MS: performing the cytotoxic test, SKA: Supervision of biological tests, NM: collaboration in identification of synthesized compounds, NE: collaboration in identifying of the structures of target compounds, manuscript preparation, MK: collaboration in manuscript preparation, AS: Collaboration in identifying the structures of target compounds, AF: Design of target compounds and supervision of the synthetic and pharmacological parts. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Vosooghi, M., Yahyavi, H., Divsalar, K. et al. Synthesis and In vitro cytotoxic activity evaluation of (E)-16-(substituted benzylidene) derivatives of dehydroepiandrosterone. DARU J Pharm Sci 21, 34 (2013). https://doi.org/10.1186/2008-2231-21-34
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2008-2231-21-34