Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Brenker, Frank E
Prior, Dave J
and
Müller, Wolfgang Friedrich
2002.
Cation ordering in omphacite and effect on deformation mechanism and lattice preferred orientation (LPO).
Journal of Structural Geology,
Vol. 24,
Issue. 12,
p.
1991.
Katerinopoulou, A.
Katerinopoulos, A.
Bieniok, A.
Knop, E.
Magganas, A.
and
Amthauer, G.
2007.
Crystal chemistry, structure analyses and phase transition experiment on an omphacite from eclogitic metagabbro from Syros island, Greece.
Mineralogy and Petrology,
Vol. 91,
Issue. 1-2,
p.
117.
Jenkins, David M.
2011.
The transition from blueschist to greenschist facies modeled by the reaction glaucophane + 2 diopside + 2 quartz = tremolite + 2 albite.
Contributions to Mineralogy and Petrology,
Vol. 162,
Issue. 4,
p.
725.
Pandolfo, Francesco
Nestola, Fabrizio
Cámara, Fernando
and
Domeneghetti, M. Chiara
2012.
New thermoelastic parameters of natural C2/c omphacite.
Physics and Chemistry of Minerals,
Vol. 39,
Issue. 4,
p.
295.
Pandolfo, Francesco
Cámara, Fernando
Domeneghetti, M. Chiara
Alvaro, Matteo
Nestola, Fabrizio
Karato, Shun-Ichiro
and
Amulele, George
2015.
Volume thermal expansion along the jadeite–diopside join.
Physics and Chemistry of Minerals,
Vol. 42,
Issue. 1,
p.
1.
Skelton, Richard
and
Walker, Andrew M.
2015.
The effect of cation order on the elasticity of omphacite from atomistic calculations.
Physics and Chemistry of Minerals,
Vol. 42,
Issue. 8,
p.
677.
Hao, Ming
Zhang, Jin S.
Pierotti, Caroline E.
Ren, Zhiyuan
and
Zhang, D.
2019.
High‐Pressure Single‐Crystal Elasticity and Thermal Equation of State of Omphacite and Their Implications for the Seismic Properties of Eclogite in the Earth's Interior.
Journal of Geophysical Research: Solid Earth,
Vol. 124,
Issue. 3,
p.
2368.
Taguchi, Tomoki
Endo, Shunsuke
Igami, Yohei
and
Miyake, Akira
2019.
A new occurrence of retrogressed eclogite from the Sanbagawa belt of southwest Japan and its significance.
Island Arc,
Vol. 28,
Issue. 5,
Castellanos-Alarcón, Oscar Mauricio
Cedeño Villarreal, Karoll Michelle
Toro Hernández, Robert Antonio
Ríos-Reyes, Carlos Alberto
Henao-Martínez, José Antonio
and
Zuluaga-Castrillón, Carlos Augusto
2022.
Crystal-Chemical and Structural Characterization of Omphacite in High-Pressure Eclogites From the Arquía Complex on Southwestern Pijao, Central Cordillera (Colombian Andes).
Frontiers in Earth Science,
Vol. 10,
Issue. ,
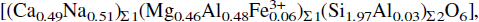 up to 1000°C. The lattice parameters were studied as a function of temperature, and their thermal expansion coefficients determined. The b and c cell edges show discontinuities as a function of temperature which are interpreted here in terms of intracrystalline cation diffusion processes. Structure refinements have been carried out using data collected at room temperature, at 800°C and at ambient conditions after cooling. The structural behaviour as a function of temperature of chemically quasi-ideal omphacites is compared with those of jadeite and diopside.
up to 1000°C. The lattice parameters were studied as a function of temperature, and their thermal expansion coefficients determined. The b and c cell edges show discontinuities as a function of temperature which are interpreted here in terms of intracrystalline cation diffusion processes. Structure refinements have been carried out using data collected at room temperature, at 800°C and at ambient conditions after cooling. The structural behaviour as a function of temperature of chemically quasi-ideal omphacites is compared with those of jadeite and diopside.



