Abstract
Background
The number of diabetes patients is steadily increasing worldwide. Consequently, the social burden of diabetes is huge, requiring urgent countermeasures. We performed an intensive survey of antidiabetic drugs approved in Japan, the United States, and the European Union
Methods
Information about approved antidiabetic drugs was obtained by searching databases of regulatory authorities in the 3 regions. Other relevant information was also obtained from publicly available literature and documents
Results
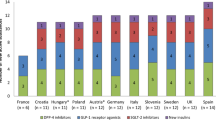
No difference in the total number and types of approved drugs among the 3 regions was found (P =.173 by log-rank test). However, the numbers of approved dipeptidyl peptidase-4 and sodium-glucose cotransporter 2 inhibitors in Japan were almost double of those in the other regions. The average sample size in clinical trials used for antidiabetic drug approval in Japan (1134 patients) was much smaller than that in the other regions (P <.001 by analysis of variance repeated measures test adjusted by the Holm method). Currently, 6 drugs with known modes of action are being developed for type 1 diabetes in Japan, whereas at the end of 2016, nearly 7-fold more products with novel modes of action were in clinical development in the United States
Conclusion
Antidiabetic drug development in Japan costs less than that in the other regions, although novel development is less active because of regulatory differences. To achieve better pharmacotherapy for diabetes, the regulatory framework requires careful consideration
Similar content being viewed by others
References
International Diabetes Federation. IDF Diabetes Atlas, 8th ed. Brussels, Belgium: International Diabetes Federation; 2017.
Health Service Bureau, Ministry of Health, Labour and Welfare. National Health and Nutrition Survey, 2016.
American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2016;39(suppl 1):S13–S22.
Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986.
Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713.
Tsukamoto K. Development of novel pharmaceutical agents for Alzheimer’s disease: The impact of regulatory initiatives in Japan and the United States. Clin Ther. 2015;37(8):1652–1660.
Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39(3):481–497.
Chapter II Guarantee of Welfare, Child Welfare Act. Law number: Act No. 164 of 1947 (Amendment: Act No. 71 of 2017).
Act on Special Child Rearing Allowance. Law number: Act No. 134 of 1964 (Amendment: Act No. 4 of 2017).
Orphan Products Development Support Program. National Institutes of Biomedical Innovation, Health and Nutrition. http://www.nibiohn.go.jp/en/activities/research-development.html. Accessed February 20, 2018.
Orphan Drug Act. Public Law 97-414.
The EU Regulation on orphan medicinal products. Regulation (EC) No 141/2000.
Manager notification No. 186. Medical Economics Division, Health Insurance Bureau, November 10, 2000.
Director, Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare. On release of the Guideline for Clinical Evaluation of Oral Hypoglycemic Agents. PFSB/ELD Notification No. 0709-1. July 2010.
Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance for Industry, Diabetes Mellitus: Developing Drugs and Therapeutic Biologics for Treatment and Prevention, February 2008.
Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance for industry, diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes, December 2008.
Committee for Medicinal Products for Human Use, European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. May 2012.
Blonde L, San Juan ZT, Bolton P. Fixed-dose combination therapy in type 2 diabetes mellitus. Endocr Pract. 2014;20:1322–1332.
Berger J, Dunn JD, Johnson MM, Karst KR, Shear WC. How drug life-cycle management patent strategies may impact formulary management. Am J Manag Care. 2016;16(suppl):S487–S495.
Notification on March 15, 2011. Pharmaceutical Evaluation Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare.
The Japan Diabetes Society. Evidence-based practice guideline for the treatment of diabetes in Japan 2013. http://www.jds.or.jp/common/fckeditor/editor/filemanager/connectors/php/transfer.php?file=/uid000025_474C323031335F656E2D30362E706466. Accessed February 20, 2017.
Anazawa T, Okajima H, Iwanaga Y, et al. Current status and the future prospects of islet transplantation. Organ Biology. 2016;23:96–98.
Anazawa T, Okajima H, Uemoto S. The current state of pancreatic islet transplantation. Japanese Journal of Transfusion and Cell Therapy. 2016;62:635–640.
Matsumoto S, Abalovich A, Wechsler C, Wynyard S, Elliott RB. Clinical benefit of islet xenotransplantation for the treatment of type 1 diabetes. EBioMedicine. 2016;12:255–262.
Sakano D, Shiraki N, Kikawa K, et al. VMAT2 identified as a regulator of late-stage β-cell differentiation. Nat Chem Biol. 2014;10(2):141–148.
Schuetz C, Anazawa T, Cross SE, et al. ß cell replacement therapy: the next 10 years. Transplantation. 2018;102:215–229.
Cnop M, Hughes SJ, Igoillo-Esteve M, et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia. 2010;53:321–330.
Scheuner D, Vander Mierde D, Song B, et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11(7):757–764.
Cunha DA, Cito M, Grieco FA, et al. Pancreatic β-cell protection from inflammatory stress by the endoplasmic reticulum proteins thrombospondin 1 and mesencephalic astrocyte-derived neurotrophic factor (MANF). J Biol Chem. 2017;292:14977–14988.
Cunha DA, Cito M, Carlsson PO, et al. Thrombospondin 1 protects pancreatic β-cells from lipotoxicity via the PERK-NRF2 pathway. Cell Death Differ. 2016;23:1995–2006.
Lee SH, Cunha D, Piermarocchi C, et al. High-throughput screening and bioinformatic analysis to ascertain compounds that prevent saturated fatty acid-induced β-cell apoptosis. Biochem Pharmacol. 2017;138:140–149.
Steele CJ, Schöttker B, Marshall AH, et al. Education achievement and type 2 diabetes-what mediates the relationship in older adults? Data from the ESTHER study: a population-based cohort study. BMJ Open. 2017;7(4):e013569.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsukamoto, K., Cnop, M., Mori, D. et al. Future Perspectives for the Treatment of Diabetes: Importance of a Regulatory Framework. Ther Innov Regul Sci 53, 535–541 (2019). https://doi.org/10.1177/2168479018795854
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479018795854