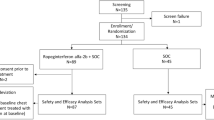
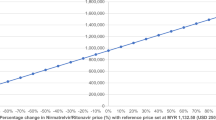
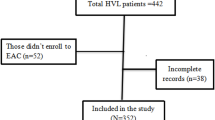
Abstract
Background
Despite wide use of nevirapine- and efavirenz-based highly active antiretroviral therapy regimens in Ethiopia, their treatment outcome has not been well studied. The objective of this study was to compare treatment outcome of nevirapine- and efavirenz-based regimens.
Methods
This retrospective cohort study was conducted on antiretroviral-naive adult patients with human immunodeficiency virus (HIV) who had started antiretroviral therapy. Study participants were excluded after treatment failure, regimen change, loss to follow-up, or transfer to other health facility. The outcomes of interest included immunologic recovery, immunologic failure, clinical failure, and treatment failure.
Results
There were 1064 HIV patients in the study; an equal proportion (1:1) from both efavirenz- and nevirapine-based regimens was included. Patients in both regimens had similar baseline CD4 cells count (P =.876). In multivariate analysis, efavirenz-based regimens showed more likelihood of immunologic recovery, whether defined as a CD4 cell count of >200 cells/mm3 (hazard ratio [HR] = 1.31 [95% CI, 1.05-1.59]), >350 cells/mm3 (HR = 1.26 [95% CI, 1.08-1.47]), or >500 cells/mm3 (HR = 1.95 [95% CI, 1.57-2.41]). Moreover, efavirenz-based regimens showed a lower hazard of treatment failure (HR = 0.66 [95% CI, 0.49-0.88]).
Conclusion
Although the finding of retrospective study should be interpreted with caution, efavirenz-based regimens were associated with superior treatment outcome.
Similar content being viewed by others
References
World Health Organization, UNICEF. Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access. Geneva, Swizerland: WHO; 2012.
World Health Organization and UNAIDS unveil plan to get 3 million AIDS patients on treatment by 2005. Ann Saudi Med. 2004;24(1):71–72.
HAPCO. Progress Report on HIV/AIDS Response. Addis Ababa, Ethiopia: HAPCO; 2012.
Zaragoza-Macias E, Cosco D, Nguyen ML, Del Rio C, Lennox J. Predictors of success with highly active antiretroviral therapy in an antiretroviral-naive urban population. AIDS Res Hum Retroviruses. 2010;26(2):133–138.
Vo TT, Ledergerber B, Keiser O, et al. Durability and outcome of initial antiretroviral treatments received during 2000–2005 by patients in the Swiss HIV Cohort Study. J Infect Dis. 2008;197(12):1685–1694.
Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368(9534):505–510.
World Health Organization. Rapid Advice: Antiretroviral Therapy for HIV Infection in Adult and Adolescent. Geneva, Switzerland: WHO; 2009.
World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach: 2010 Revision. Geneva, Switzerland: WHO; 2010.
Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society–USA panel. JAMA. 2010;304(3):321–333.
Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4):387–402.
US Department of Health and Human Services. adult and adolescent ARV guidelines AIDSinfo. http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/11/what-to-start. Accessed February 12, 2013.
Gazzard BG, on behalf of the BTGWG. British HIV Association guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med. 2008;9(8):563–608.
Nunez M, Soriano V, Martin-Carbonero L, et al. SENC (Spanish efavirenz vs. nevirapine comparison) trial: a randomized, open-label study in HIV-infected naive individuals. HIV Clin Trials. 2002;3(3):186–194.
van Leth F, Phanuphak P, Ruxrungtham K, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet. 2004;363(9417):1253–1263.
Mbuagbaw LC, Irlam JH, Spaulding A, Rutherford GW, Siegfried N. Efavirenz or nevirapine in three-drug combination therapy with two nucleoside-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral-naive individuals. Cochrane Database Syst Rev. 2010;(12):CD004246.
The effect of efavirenz versus nevirapine-containing regimens on immunologic, virologic and clinical outcomes in a prospective observational study. AIDS. 2012;26(13):1691–1705.
Braithwaite RS, Kozal MJ, Chang CC, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS. 2007;21(12):1579–1589.
Cozzi-Lepri A, Phillips AN, d’Arminio Monforte A, et al. Virologic and immunologic response to regimens containing nevirapine or efavirenz in combination with 2 nucleoside analogues in the Italian Cohort Naive Antiretrovirals (I.Co.N.A.) study. J Infect Dis. 2002;185(8):1062–1069.
Bannister WP, Ruiz L, Cozzi-Lepri A, et al. Comparison of genotypic resistance profiles and virological response between patients starting nevirapine and efavirenz in EuroSIDA. AIDS. 2008;22(3):367–376.
Pillay P, Ford N, Shubber Z, Ferrand RA. Outcomes for efavirenz versus nevirapine-containing regimens for treatment of HIV-1 infection: a systematic review and meta-analysis. PLoS ONE. 2013;8(7):e68995.
Nachega JB, Hislop M, Dowdy DW, et al. Efavirenz versus nevirapine-based initial treatment of HIV infection: clinical and virological outcomes in Southern African adults. AIDS. 2008;22(16):2117–2125.
Keiser P, Nassar N, White C, Koen G, Moreno S. Comparison of nevirapine- and efavirenz-containing antiretroviral regimens in antiretroviral-naive patients: a cohort study. HIV Clin Trials. 2002;3(4):296–303.
de Beaudrap P, Etard JF, Gueye FN, et al. Long-term efficacy and tolerance of efavirenz- and nevirapine-containing regimens in adult HIV type 1 Senegalese patients. AIDS Res Hum Retroviruses. 2008;24(6):753–760.
Wester CW, Thomas AM, Bussmann H, et al. Non-nucleoside reverse transcriptase inhibitor outcomes among combination antiretroviral therapy-treated adults in Botswana. AIDS. 2010;24 (suppl 1): S27–S36.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kedir, M.S., Gemeda, D.H. & Suleman, S. Treatment Outcomes of Nevirapine- Versus Efavirenz-Based Highly Active Antiretroviral Therapy Regimens Among Antiretroviral-Naive Adult Patients in Ethiopia: A Cohort Study. Ther Innov Regul Sci 49, 443–449 (2015). https://doi.org/10.1177/2168479014565472
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479014565472