Abstract
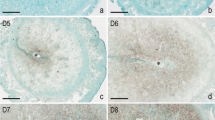
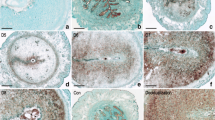
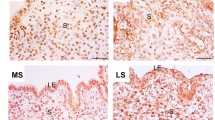
Uterine decidualization is crucial for placenta formation and pregnancy maintenance. Although previous studies have reported that high mobility group box 3 (Hmgb3) is involved in the regulation of cellular proliferation and differentiation, little is known regarding its physiological role in uterine decidualization. Here, in situ hybridization result exhibited a dynamic expression pattern of Hmgb3 messenger RNA (mRNA) during early gestation, and it was mainly localized to the decidua on days 6 to 8 of gestation. Consistently, elevated Hmgb3 expression was noted in the decidualizing stromal cells after intraluminal oil infusion. In uterine luminal epithelium of ovariectomized mice, estrogen induced the accumulation of Hmgb3 mRNA, which was dependent on the existence of implanting blastocyst. Simultaneously, Hmgb3 could stimulate the proliferation of uterine stromal cells and promote the expression of Prl8a2, a reliable marker for stromal cell differentiation. Further analysis evidenced that Hmgb3 might modulate the expression of pleiotropin (Ptn) in uterine stromal cells. Moreover, silencing of Ptn could impede the upregulation of Prl8a2 elicited by Hmgb3 overexpression, while overexpression of Ptn reversed the repressive effects of Hmgb3 siRNA on Prl8a2 expression. Collectively, Hmgb3 may direct uterine decidualization through targeting Ptn.
Similar content being viewed by others
References
Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–1767.
Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7(3):185–199.
Zhang S, Lin H, Kong S, et al. Physiological and molecular determinants of embryo implantation. Mol Aspects Med. 2013;34(5):939–980.
Vaccari T, Beltrame M, Ferrari S, Bianchi ME. Hmg4, a new member of the Hmg1/2 gene family. Genomics. 1998;49(2):247–252.
Hock R, Furusawa T, Ueda T, Bustin M. HMG chromosomal proteins in development and disease. Trends Cell Biol. 2007;17(2):72–79.
Lv Z, Zhang Z, Wei Z, et al. HMGB3 modulates ROS production via activating TLR cascade in Apostichopus japonicus. Dev Comp Immunol. 2017;77:128–137.
Taniguchi N, Kawakami Y, Maruyama I, Lotz M. HMGB proteins and arthritis. Hum Cell. 2018;31(1):1–9.
Li M, Cai Y, Zhao H, et al. Overexpression of HMGB3 protein promotes cell proliferation, migration and is associated with poor prognosis in urinary bladder cancer patients. Tumour Biol. 2015;36(6):4785–4792.
Zhang Z, Chang Y, Zhang J, et al. HMGB3 promotes growth and migration in colorectal cancer by regulating WNT/β-catenin pathway. Plos One. 2017;12(7):e0179741.
Guo S, Wang Y, Gao Y, et al. Knockdown of high mobility group-Box 3 (HMGB3) expression inhibits proliferation, reduces migration, and affects chemosensitivity in gastric cancer cells. Med Sci Monit. 2016;22:3951–3960.
Nemeth MJ, Curtis DJ, Kirby MR, et al. Hmgb3: an HMG-box family member expressed in primitive hematopoietic cells that inhibits myeloid and B-cell differentiation. Blood. 2003;102(4):1298–1306.
Nemeth MJ, Cline AP, Anderson SM, Garrett-Beal LJ, Bodine DM. Hmgb3 deficiency deregulates proliferation and differentiation of common lymphoid and myeloid progenitors. Blood. 2005;105(2):627–634.
Nemeth MJ, Kirby MR, Bodine DM. Hmgb3 regulates the balance between hematopoietic stem cell self-renewal and differentiation. Proc Natl Acad Sci U S A. 2006;103(37):13783–13788.
Fan QW, Muramatsu T, Kadomatsu K. Distinct expression of midkine and pleiotrophin in the spinal cord and placental tissues during early mouse development. Dev Growth Differ. 2000;42(2):113–119.
Muramatsu H, Zou P, Kurosawa N, et al. Female infertility in mice deficient in midkine and pleiotrophin, which form a distinct family of growth factors. Genes Cells. 2006;11(12):1405–1417.
Yu HF, Tao R, Yang ZQ, Wang K, Yue ZP, Guo B. Ptn functions downstream of C/EBPβ to mediate the effects of cAMP on uterine stromal cell differentiation through targeting Hand2 in response to progesterone. J Cell Physiol. 2018;233(2):1612–1626.
Chung HW, Wen Y, Choi EA, et al. Pleiotrophin (PTN) and midkine (MK) mRNA expression in eutopic and ectopic endometrium in advanced stage endometriosis. Mol Hum Reprod. 2002;8(4):350–355.
Sethi G, Kwon Y, Burkhalter RJ, et al. PTN signaling: components and mechanistic insights in human ovarian cancer. Mol Carcinog. 2015;54(12):1772–1785.
Yu HF, Yue ZP, Wang K, et al. Gja1 acts downstream of Acvr1 to regulate uterine decidualization via Hand2 in mice. J Endocrinol. 2017;233(2):145–157.
Dey SK, Lim H, Das SK, et al. Molecular cues to implantation. Endocr Rev. 2004;25(3):341–373.
Li DD, Zhao SY, Yang ZQ, et al. Hmgn5 functions downstream of Hoxa10 to regulate uterine decidualization in mice. Cell Cycle. 2016;15(20):2792–2805.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Authors’ Note
Kai Wang and Yun-Hou Yin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, K., Yin, YH., Yang, ZQ. et al. Hmgb3 Induces the Differentiation of Uterine Stromal Cells Through Targeting Ptn. Reprod. Sci. 26, 891–899 (2019). https://doi.org/10.1177/1933719118792098
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719118792098