Abstract
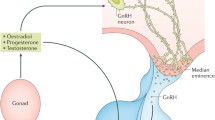
Proper gonadal function requires coordinated (feedback) interactions between the gonads, adenohypophysis, and brain: the gonads elaborate sex steroids (progestins, androgens, and estrogens) and proteins (inhibin-activin family) during gamete development. In both sexes, the brain-pituitary gonadotrophin-regulating interaction is coordinated by estradiol through its opposing actions on pituitary gonadotrophs (sensitization of the response to gonadotrophin-releasing hormone [GnRH]) versus hypothalamic neurons (inhibition of GnRH secretion). This dynamic tension between the gonadotrophs and the GnRH cells in the brain regulates the circulating gonadotrophins and is termed reciprocal/negative feedback. In females, reciprocal/negative feedback dominates ∼ 90% of the ovarian cycle. In a spectacular exception, the dynamic tension is broken during the surge of circulating estrogen that marks follicle and oocyte(s) maturation. The cause is an estradiol-induced disinhibition of the GnRH neurons that releases GnRH secretion to the highly sensitized pituitary gonadotrophs that in turn release the gonadotrophin surge (the estrogen-induced gonadotrophin surge [EIGS], also known as positive feedback). Studies during the past 4 decades have shown this disinhibition to result from estrogen-induced synaptic plasticity (EISP), including a reversible ∼ 50% loss in arcuate nucleus synapses. The disinhibited GnRH secretion occurs during maximal gonadotroph sensitization and results in the EIGS. Specific immunoneutralization of estradiol blocks the EISP and EIGS. The EISP is accompanied by increases in insulinlike growth factor 1, polysialylated neural cell adhesion molecule, and ezrin, 3 proteins that the authors believe are the links between estrogen-induced astroglial extension and the EISP that releases GnRH secretion at the moment of maximal sensitization of the pituitary gonadotrophs. The result is the paradoxical surge of gonadotrophins at the peak of ovarian estrogen secretion and the triggering of ovulation. This enhanced understanding of the mechanics of gonadotrophin control clarifies elements of the involved feedback loops and opens the way to a better understanding of the neurobiology of reproduction.
Similar content being viewed by others
References
Harris GW, Naftolin F. The hypothalamus and control of ovulation. Br Med Bull. 1970;26:3–9.
Knobil E., Plant TM, Wildt L., Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science. 1980;207: 1371–1373.
Naftolin F., Zreik T., Garcia-Segura L., Horvath T. Neuroendocrine control of reproduction. In: Seifer DB, Samuels P, Kniss DA, eds. The Physiologic Basis of Gynecology and Obstetrics. Philadelphia, Pa: Lippincott, Williams and Wilkins; 2001:63–74.
Burger HG Evidence for a negative feedback role of inhibin in follicle stimulating hormone regulation in women. Hum Reprod. 1993;8(suppl 2):129–132.
Nillius SJ, Wide L. Variation in LH and FSH response to LH-releasing hormone during the menstrual cycle. J Obstet Gynaecol Br Commonw. 1972;79:865–873.
Corker CS, Naftolin F., Exley D. Interrelationship between plasma luteinizing hormone and oestradiol in the human menstrual cycle. Nature. 1969;222:1063.
Judd SJ, Rakoff JS, Yen SS Inhibition of gonadotropin and prolactin release by dopamine: effect of endogenous estradiol levels. J Clin Endocrinol Metab. 1978;47:494–498.
Ropert JF, Quigley ME, Yen SS The dopaminergic inhibition of LH secretion during the menstrual cycle. Life Sci. 1984; 34:2067–2073.
Kalra SP, Kalra PS Neural regulation of luteinizing hormone secretion in the rat. Endocr Rev. 1983;4:311–351.
Gore AC, Terasawa E. Neural circuits regulating pulsatile luteinizing hormone release in the female guinea-pig: opioid, adrenergic and serotonergic interactions. J Neuroendocrinol. 2001;13:239–248.
Malyala A., Kelly MJ, Ronnekleiv OK Estrogen modulation of hypothalamic neurons: activation of multiple signaling pathways and gene expression changes. Steroids. 2005;70: 397–406.
Rossmanith WG, Mortola JF, Yen SS Role of endogenous opioid peptides in the initiation of the midcycle luteinizing hormone surge in normal cycling women. J Clin Endocrinol Metab. 1988;67:695–700.
Ferin M., Dyrenfurth I., Cowchock S., Warren M., Wiele RL Active immunization to 17 beta-estradiol and its effects upon the reproductive cycle of the rhesus monkey. Endocrinology. 1974;94:765–776.
Ferin M., Tempone A., Zimmering PE, Van de Wiele RL Effect of antibodies to 17beta-estradiol and progesterone on the estrous cycle of the rat. Endocrinology. 1969;85:1070–1078.
Naftolin F., Mor G., Horvath TL, et al. Synaptic remodeling in the arcuate nucleus during the estrous cycle is induced by estrogen and precedes the preovulatory gonadotropin surge. Endocrinology. 1996;137:5576–5580.
Zsarnovszky A., Horvath TL, Garcia-Segura LM, Horvath B., Naftolin F. Oestrogen-induced changes in the synaptology of the monkey (Cercopithecus aethiops) arcuate nucleus during gonadotropin feedback. J Neuroendocrinol. 2001;13:22–28.
Fernandez-Galaz MC, Morschl E., Chowen JA, Torres-Aleman I., Naftolin F., Garcia-Segura LM Role of astroglia and insulin-like growth factor-I in gonadal hormone-dependent synaptic plasticity. Brain Res Bull. 1997;44:525–531.
Garcia-Segura LM, Canas B., Parducz A., et al. Estradiol promotion of changes in the morphology of astroglia growing in culture depends on the expression of polysialic acid of neural membranes. Glia. 1995;13:209–216.
Garcia-Segura LM, McCarthy MM Minireview: role of glia in neuroendocrine function. Endocrinology. 2004;145:1082–1086.
MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302.
Pau KY, Spies HG Neuroendocrine signals in the regulation of gonadotropin-releasing hormone secretion. Chin J Physiol. 1997;40:181–196.
Soendoro T., Diamond MP, Pepperell JR, Naftolin F. The in vitro perifused rat ovary: I. Steroid secretion in response to ramp and pulsatile stimulation with luteinizing hormone and follicle stimulating hormone. Gynecol Endocrinol. 1992;6:229–238.
Rebar R., Perlman D., Naftolin F., Yen SS The estimation of pituitary luteinizing hormone secretion. J Clin Endocrinol Metab. 1973;37:917–927.
Naftolin F., Ryan KJ, Davies IJ, et al. The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res. 1975;31:295–319.
Schindler AE Steroid metabolism of fetal tissues. II. Conversion of androstenedione to estrone. Am J Obstet Gynecol. 1975;123: 265–268.
McArdle CA, Schomerus E., Groner I., Poch A. Estradiol regulates gonadotropin-releasing hormone receptor number, growth and inositol phosphate production in alpha T3-1 cells. Mol Cell Endocrinol. 1992;87:95–103.
Gordan JD, Attardi BJ, Pfaff DW Mathematical exploration of pulsatility in cultured gonadotropin-releasing hormone neurons. Neuroendocrinology. 1998;67:2–17.
Moenter SM, Caraty A., Locatelli A., Karsch FJ Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology. 1991;129:1175–1182.
Lopez FJ, Merchenthaler IJ, Moretto M., Negro-Vilar A. Modulating mechanisms of neuroendocrine cell activity: the LHRH pulse generator. Cell Mol Neurobiol. 1998;18:125–146.
Terasawa E., Schanhofer WK, Keen KL, Luchansky L. Intracellular Ca(2+) oscillations in luteinizing hormone— releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci. 1999;19: 5898–5909.
Leranth C., MacLusky N., Naftolin F. Interconnections Between Neurotransmitter- and Neuropeptide-Containing Neurons Involved in Gonadotropin Release in the Rat. New York: Plenum; 1986.
Lawson MA, Macconell LA, Kim J., Powl BT, Nelson SB, Mellon PL Neuron-specific expression in vivo by defined transcription regulatory elements of the GnRH gene. Endocrinology. 2002;143:1404–1412.
Witkin JW, Xiao E., Popilskis S., Ferin M., Silverman AJ FOS expression in the gonadotropin-releasing hormone (GnRH) neuron does not increase during the ovarian steroid-induced GnRH surge in the rhesus monkey. Endocrinology. 1994;135: 956–961.
Mizuno M., Terasawa E. Search for neural substrates mediating inhibitory effects of oestrogen on pulsatile luteinising hormone—releasing hormone release in vivo in ovariectomized female rhesus monkeys (Macaca mulatta). J Neuroendocrinol. 2005; 17:238–245.
Clayton RN, Solano AR, Garcia-Vela A., Dufau ML, Catt KJ Regulation of pituitary receptors for gonadotropin-releasing hormone during the rat estrous cycle. Endocrinology. 1980; 107:699–706.
Taga M., Minaguchi H., Kigawa T., Sakamoto S. Effects of sex steroid hormones on rat anterior pituitary LH-RH receptor. Nippon Sanka Fujinka Gakkai Zasshi. 1982;34:627–633.
Emons G., Hoffmann HG, Brack C., et al. Modulation of gonadotropin-releasing hormone receptor concentration in cultured female rat pituitary cells by estradiol treatment. J Steroid Biochem. 1988;31:751–756.
Naftolin F., Yen SS, Tsai CC Rapid cycling of plasma gonadotrophins in normal men as demonstrated by frequent sampling. Nat New Biol. 1972;236:92–93.
Yen SS, Tsai CC, Naftolin F., Vandenberg G., Ajabor L. Pulsatile patterns of gonadotropin release in subjects with and without ovarian function. J Clin Endocrinol Metab. 1972;34:671–675.
Matsumoto A., Arai Y., Urano A., Hyodo S. Molecular basis of neuronal plasticity to gonadal steroids. Funct Neurol. 1995;10:59–76.
Witkin JW, Ferin M., Popilskis SJ, Silverman AJ Effects of gonadal steroids on the ultrastructure of GnRH neurons in the rhesus monkey: synaptic input and glial apposition. Endocrinology. 1991;129:1083–1092.
Woolley CS Effects of oestradiol on hippocampal circuitry. Novartis Found Symp. 2000;230:173–180.
Naftolin F., Leranth C., Garcia-Segura L. Ultrastructural changes in hypothalamic cells during estrogen-induced gonadotrophin feedback. Neuroprotocols. 1992;1:16–26.
Naftolin F., Garcia-Segura LM, Keefe D., Leranth C., Maclusky NJ, Brawer JR Estrogen effects on the synaptology and neural membranes of the rat hypothalamic arcuate nucleus. Biol Reprod. 1990;42:21–28.
Brawer JR, Naftolin F., Martin J., Sonnenschein C. Effects of a single injection of estradiol valerate on the hypothalamic arcuate nucleus and on reproductive function in the female rat. Endocrinology. 1978;103:501–512.
Garcia-Segura LM, Chowen JA, Duenas M., Torres-Aleman I., Naftolin F. Gonadal steroids as promoters of neuro-glial plasticity. Psychoneuroendocrinology. 1994;19:445–453.
Parducz A., Perez J., Garcia-Segura LM. Estradiol induces plasticity of gabaergic synapses in the hypothalamus. Neuroscience. 1993;53:395–401.
Brown-Grant K., Exley D., Naftolin F. Peripheral plasma oestradiol and luteinizing hormone concentrations during the oestrous cycle of the rat. J Endocrinol. 1970;48:295–296.
Garcia-Segura LM, Hernandez P., Olmos G., Tranque PA, Naftolin F. Neuronal membrane remodelling during the oestrus cycle: a freeze-fracture study in the arcuate nucleus of the rat hypothalamus. J Neurocytol. 1988;17:377–383.
Olmos G., Naftolin F., Perez J., Tranque PA, Garcia-Segura LM. Synaptic remodeling in the rat arcuate nucleus during the estrous cycle. Neuroscience. 1989;32:663–667.
Naftolin F., Bruhlmann-Papazyan M., Baetens D., Garcia-Segura LM Neurons with whorl bodies have increased numbers of synapses. Brain Res. 1985;329:289–293.
Naftolin F., MacLusky NJ, Leranth CZ, Sakamoto HS, Garcia-Segura LM The cellular effects of estrogens on neuroendocrine tissues. J Steroid Biochem. 1988;30:195–207.
Naftolin F., Leranth C., Perez J., Garcia-Segura LM. Estrogen induces synaptic plasticity in adult primate neurons. Neuroendocrinology. 1993;57:935–939.
Horvath TL, Diano S., van den Pol AN Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci. 1999;19: 1072–1087.
Duenas M., Luquin S., Chowen JA, Torres-Aleman I., Naftolin F., Garcia-Segura LM Gonadal hormone regulation of insulinlike growth factor-I-like immunoreactivity in hypothalamic astroglia of developing and adult rats. Neuroendocrinology. 1994; 59:528–538.
Brawer JR The fine structure of the ependymal tanycytes at the level of the arcuate nucleus. J Comp Neurol. 1972;145:25–41.
Jakab RL, Horvath TL, Leranth C., Harada N., Naftolin F. Aromatase immunoreactivity in the rat brain: gonadectomysensitive hypothalamic neurons and an unresponsive “limbic ring” of the lateral septum-bed nucleus-amygdala complex. J Steroid Biochem Mol Biol. 1993;44:481–498.
Theodosis DT, Bonfanti L., Olive S., Rougon G., Poulain DA Adhesion molecules and structural plasticity of the adult hypothalamo-neurohypophysial system. Psychoneuroendocrinology. 1994;19:455–462.
Seifer D., Roa L., Keefe D., et al. Increasing hypothalamic arcuate nucleus glial peroxidase activity in aging female rats is reduced by an antiestrogen and a gonadotropin-releasing hormone agonist. Menopause. 1994;2:83–90.
Joseph SA, Knigge KM The endocrine hypothalamus: recent anatomical studies. Res Publ Assoc Res Nerv Ment Dis. 1978; 56:15–47.
Garcia-Segura LM, Naftolin F., Hutchison JB, Azcoitia I., Chowen JA Role of astroglia in estrogen regulation of synaptic plasticity and brain repair. J Neurobiol. 1999;40:574–584.
Garcia-Segura LM, Baetens D., Naftolin F. Sex differences and maturational changes in arcuate nucleus neuronal plasma membrane organization. Brain Res. 1985;351:146–149.
Olmos G., Aguilera P., Tranque P., Naftolin F., Garcia-Segura LM Estrogen-induced synaptic remodelling in adult rat brain is accompanied by the reorganization of neuronal membranes. Brain Res. 1987;425:57–64.
Garcia-Segura LM, Perez J., Tranque PA, Olmos G., Naftolin F. Sex differences in plasma membrane concanavalin A binding in the rat arcuate neurons. Brain Res Bull. 1989;22:651–655.
Derouiche A., Frotscher M. Peripheral astrocyte processes: monitoring by selective immunostaining for the actin-binding ERM proteins. Glia. 2001;36:330–341.
Bretscher A., Edwards K., Fehon RG ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002; 3:586–599.
Song J., Fadiel A., Edusa V., et al. Estradiol-induced ezrin overexpression in ovarian cancer: a new signaling domain for estrogen. Cancer Lett. 2005;220:57–65.
Garcia-Segura LM, Duenas M., Fernandez-Galaz MC, et al. Interaction of the signalling pathways of insulin-like growth factor-I and sex steroids in the neuroendocrine hypothalamus. Horm Res. 1996;46:160–164.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by NIH HD 13587 and NS36111 (F.N.), NSF IBN-IBN-9728581 (T.L.H.), and SAF 2005-00272, Ministerio de Educacion y Ciencia, Spain (L.-M.G.S.).
Rights and permissions
About this article
Cite this article
Naftolin, F., Garcia-Segura, L.M., Horvath, T.L. et al. Estrogen-Induced Hypothalamic Synaptic Plasticity and Pituitary Sensitization in the Control of the Estrogen-Induced Gonadotrophin Surge. Reprod. Sci. 14, 101–116 (2007). https://doi.org/10.1177/1933719107301059
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719107301059