Abstract
Dobutamine is a synthetic catecholamine used in the treatment of different cardiac pathologies associated with organic heart diseases, myocardial infarction and cardiac surgery.
The ESR spectra of M2+-o-semiquinone radicals, obtained by the oxidation of dobutamine HCl with horseradish peroxidase and hydrogea peroxide in the presence of several bivalent metal ions of IIA (Mg, Ca, Sr, Ba) and IIB (Zn, Cd) groups, have been reported.
ESR experimental spectra have been simulated and completely characterized. The dependence of the proton hyperfine coupling constant values on the complexing ion radius has been discussed together with the differences in the radicals concentrations.
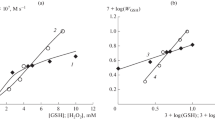
Furthermore, the formation and decay kinetics of Zn2+-dobutamine-o-semiquinone have been analyzed at varying H2O2 concentrations, and the corresponding kinetic constants have been calculated.
Similar content being viewed by others
References
N. Wiener. In: Goodman and Gilman’s, The Pharmacological Basis of Therapeutics, Macmillan Publishing Company, New York, p. 134, 1991.
N.W. Robie, D.O. Nutter, C. Moody, and J.L. McNay, Circ Res., 34, 663 (1974).
S.F. Vatner, R.J. McRitchie, P.R. Maroko, T.A. Patrick, and E. Braunwald, J. Clin. Invest., 54, 563 (1974).
L.I. Goldberg, Y.-Y. Hsieh, and L. Resnekov, Prog. Cardiovasc. Dis., 19, 327 (1977).
G.A. Holloway and E.L. Frederickson, Anesth. Analg., 53, 616 (1974).
D.W. McKennon and R.E. Kates, J. Pharm. Sci., 67, 1756 (1978).
H. Lingeman, J.A. Haverhals, H.J.E.M. Reeuwijk, U.R. Tjaden, and J. van der Greef, Chromatogr, 24, 886 (1987).
M.E. El-Kommos, Analyst 112, 101 (1987).
M.E. El-Kommos, Analyst 108, 380 (1983).
M.E. El-Kommos, F.A. Mohamed, and A.A.S. Khedr, J. Assoc. Off. Anal. Chem., 73, 516 (1990).
H.S. Mason, J. Biol. Chem., 172, 83 (1948).
T.E. Young, J.R. Griswold, M.H. Hulbert, J. Org. Chem. 39, 1980 (1974).
W. Korytowski, T. Sarna, B. Kalyanaramanan, and R.C. Sealy, Biochim. Biophys. Acta 924, 383 (1987).
I.E. Blasig, B. Ebert, T. Hanke, and H. Lowe Pharmazie 43, 139 (1988).
B. Kalyanaraman, Meth. Enzymol. 186, 333 (1990).
R.P. Ferrari, E. Laurenti, L. Casella, and S. Poli, Spectrochim. Acta 49A, 1261 (1993).
R.P. Ferrari and E. Laurenti, J. Inorg. Biochem. (1995), in press.
C.C. Felix and R.C. Sealy, J. Am. Chem. Soc., 103, 2831 (1981).
R.P. Ferrari, E. Laurenti, and E.M. Ghibaudi, Eur. J. Biochem. (1995), submitted.
B. Kalyanaraman and R.C. Sealy, Biochim. Biophys. Res. Comm., 106, 119 (1982).
T.J. Stone and W.A. Waters, J. Chem. Soc. 1486 (1965).
A.J. Dobbs, B.C. Gilbert, and R.O.C. Norman, J. Chem. Soc., Perkin Trans., 2 2053 (1972).
D.R. Eaton, Inorg. Chem. 3, 1268 (1964).
G.F. Pedulli, A. Alberti, L. Testaferri, and M. Tiecco, J. Chem. Soc. Perkin Trans. 2 1701 (1974).
B. Kalyanaraman, C.C. Felix, and R.C. Sealy, J. Biol. Chem. 259, 7584 (1984).
N. Oshino, B. Chance, H. Sies, and T. Bucher, Arch. Biochem. Biophys. 154, 117 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ferrari, R.P., Laurenti, E., Ghibaudi, E.M. et al. ESR characterization and kinetics of the enzymatically obtained M(II)-dobutamine-o-semiquinone system. Res. Chem. Intermed. 22, 459–468 (1996). https://doi.org/10.1163/156856796X00665
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856796X00665