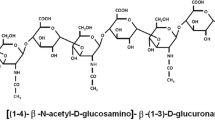
Abstract
Objective: To study the effect of hyaluronan on cell adhesion and recruitment both in vitro and in vivo, since hyaluronan both inhibits restenosis and is anti-inflammatory. When administered to animals undergoing angioplasty the recruitment of cells into the restenotic plaque is inhibited, as well as into inflammatory lesions. The recent discovery that ICAM-1 binds hyaluronan and exhibits the B(X(7))B HA binding motif, led us also to investigate whether cell adhesion could be modulated by hyaluronan.
Materials and methods: Human neutrophils were adhered to human umbilical vein (HUVEC) or Ea.hy.926 HUVEC cells stimulated with phorbol myristate acetate (PMA) or tumour necrosis factor (TNFα). Neutrophil binding in vivo utilized FMLP-stimulated hamster cheek pouch post-capillary venules.
Results: Hyaluronan inhibited human neutrophil adhesion to both PMA and TNFα-stimulated HUVEC. Ea.hy.926 human immortal HUVECs expressed ICAM-1 in response to TNFα and PMA. E-selectin was also upregulated by 6 h with TNFα but not significantly with PMA. TNFα induced CD44 expression within 4 h, but PMA not significantly up to 6 h. However, specific binding of [125I]hyaluronan to Ea.hy.926 cells was increased by PMA-stimulation at 4 h. Neutrophil adhesion to PMA-stimulated Ea.hy.926 HUVECs was inhibited in a concentration dependent fashion by both anti-ICAM-1 and hyaluronan (1 ng/ml-10 µg/ml) at 4 h. At 1 mg/ml adhesion was stimulated by hyaluronan. Hyaluronan had no effect on neutrophil adhesion to resting Ea.hy.926 cells. Hyaluronan (25 mg/kg, i.v.) inhibited cell adhesion to FMLP-stimulated post capillary venules of the hamster cheek pouch, whilst leaving cell rolling unaffected.
Conclusions: These results show that hyaluronan, at concentrations below those where intramolecular associations occur, binds selectively to stimulated endothelial cells and inhibits neutrophil adhesion in vitro and in vivo via a mechanism which may involve molecules other than CD44, such as ICAM-1.
Similar content being viewed by others
References
Albleda, S. M. (1991). Endothelium and epithelial cell adhesion molecules, Am. J. Respir. Cell Mol. Biol. 4, 195–203.
Austin, G. E., Ratcliff, N. B., Hollman, J., Tabei, S. and Phillips, D. F. (1985). Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty, J. Am. Coll. Cardiol. 6, 369–375.
Bath, P. M., Booth, R. F. and Hassall, D. G. (1989). Monocyte-lymphocyte discrimination in a new microtitre-based adhesion assay, J. Immunol. Methods 118, 59–65.
Bellitos, P. C., Hildreth, J. E. K. and August, J. T. (1990). Homotypic cell aggregation induced by anti-CD44 (PGP-1) monoclonal antibodies and related to CD44 (PGP-1) expression, J. Immunol. 144, 1661–1670.
Bevilacqua, M. P. (1993). Endothelial-leucocyte adhesion molecules, Annu. Rev. Immunol. 11, 767–804.
Chen, Q., Cai, S., Shadrach, K. G., Prestwich, G. D. and Hollyfield, J. G. (2004). Spacrcan binding to hyaluronan and other glycosaminoglycans. Molecular and biochemical studies, J. Biol. Chem. 279, 23142–23150.
Clagett, G. P., Robinowitz, M., Yourke, J. R., Fisher, D. F., Fry, R. E., Myers, S. I., Lee, E. I., Collins, G. J. and Virmani, R. (1986). Morphogenesis and clinicopathologic characteristics of recurrent carotid disease, J. Vasc. Surg. 3, 10–23.
Clausell, N., Lima, V. C., Molossi, S., Turley, E., Gotleib, A., Rabinovic, M., Liu, P. and Adelman, A. (1993). Restenosis following directional coronary athrectomy associated with inflammation and increased fibronectin, J. Am. Coll. Cardiol. 124A, 734–736.
Clowes, A. W., Reidy, M. A. and Clowes, M. M. (1989). Kinetics of cellular proliferation after arterial injury. I. Smooth muscle cell growth in the absence of endothelium, Lab. Invest. 49, 327–333.
Corrado, E. M., Peluso, G. F., Gigliotti, S., De Durante C. D., Palmier, D., Savioa, M., Oriani, G. O. and Tajana, G. F. (1995). The effects of intra-articular administration of hyaluronic acid on osteoarthritis of the knee: A clinical study with immunological and biochemical evaluations, Eur. J. Rheumatol. Inflamm. 15, 47–56.
Dwivedi, A. and Carrier, M. J. (2000). Oxidised LDL-mediated monocyte adhesion to endothelial cells does not involve NFκB, Biochem. Biophys. Res. Commun. 284, 239–244.
Ferns, G. A. A., Konneh, M., Rutherford, C., Woolaghan, E. and Anggard, E. E. (1996). Hyaluronan inhibits neuointimal macrophage influx after balloon-catheter induced injury in the cholesterol fed rabbit, Atherosclerosis 114, 157–164.
Forrester, J. V. and Lackie, J. M. (1981). Effect of hyaluronic acid on neutrophil adhesion, J. Cell Sci. 50, 329–344.
Gallatin, W. M., Rosenman, S. J., Ganji, A., St. John, T. P., Cochrane, C. G. and Gimbrone Jr., M. A. (1990). Structure-function relationships of the CD44 class of glycoproteins, in: Cellular and Molecular Mechanisms of Inflammation, Vol. 2. Vascular Adhesion Molecules, Cochrane, C. G. and Gimbrone Jr., M. A. (Eds), pp. 131–150. Academic Press, San Diego, CA.
Gustafson, S., Bjorkman, T. and Westlin, J. E. (1994). Labelling of high molecular weight hyaluronan with I-125 tyrosine — studies in vitro and in vivo in the rat, Glycoconjug. J. 11, 608–613.
Håkansson, L. and Venge, P. (1987). The molecular basis of the hyaluronic acid mediated stimulation of granulocyte function, J. Immunol. 138, 4347–4352.
Håkansson, L., Hallgren, R. and Venge, P. (1980). Regulation of granulocyte function by hyaluronic acid. In vitro and in vivo effects on phagocytosis, locomotion and metabolism, J. Clin. Invest. 66, 298–305.
Hilleman, R. E., Fromm, J. R., Weiler, J. M. and Linhardt, R. J. (1998). Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins, Bioessays 20, 156–167.
Ialenti, A. and DiRosa, M. (1994). Hyaluronic acid modulates acute and chronic inflammation. Agents Actions 43, 44–47.
Kuijpers, T. W., Hakkert, B. C., Hart, M. H. L. and Roos, D. (1992). Neutrophil migration across monolayers of cytokine-prestimulated endothelial cells — a role for platelet-activating factor and IL-8, J. Cell Biol. 117, 565–572.
Lisignoli, G., Grassi, F., Zini, N., Toneguzzi, S., Piacentinti, A., Guidolin, D., Bevilacqua, C. and Facchini, A. (2001). Anti-Fas-induced apoptosis in chondrocytes reduced by hyaluronan: evidence for CD44 and CD54 (intercellular adhesion molecule 1) involvement, Arthritis Rheum. 44, 1800–1807.
Mancuso, F., Flower, R. J. and Perretti, M. (1995). Leukocyte transmigration, but not rolling or adhesion, is selectively inhibited by dexamethasone in the hamster post-capillary venule — involvement of endogenous lipocortin-1, J. Immunol. 155, 377–386.
McCourt, P. A. G., Ek, B., Forsberg, N. and Gustafson, S. (1994). Intercellular adhesion molecule-1 is a cell surface receptor for hyaluronan, J. Biol. Chem. 269, 30081–30084.
Moore, A. R., Chander, C. L., Hanahoe, T. H. P., Howat, D. W., Desa, F. M., Colville-Nash, P. R. and Willoughby, D. A. (1989). The chemotactic properties of cartilage glycosaminoglycans for polymorphonuclear neutrophils, Int. J. Tissue React. 11, 301–307.
Oda, T. and Katori, M. (1992). Inhibition site of dexamethasone on extravasation of polymorphonuclear leukocytes in the hamster cheek pouch, J. Leukocyte Biol. 52, 337–342.
Samuelsson, C. and Gustafson, S. (1998). Studies on the interaction between hyaluronan and a rat colon cancer cell line, Glycoconj. J. 15, 169–175.
Savani, R. C. and Turley, E. A. (1995). The role of hyaluronan and its receptors in restenosis after balloon angioplasty — development of a potential therapy, Int. J. Tiss. React. 17, 141–151.
Scott, J. E. (1989). Secondary structures in hyaluronan solutions: chemical and biological implications, Ciba Found. Symp. 143, 6–20.
Scott, J. E., Cummings, C., Brass, A. and Chen, Y. (1991). Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation, Biochem. J. 274, 699–705.
Springer, T. A. (1990). Adhesion receptors of the immune system. Nature 346, 425–434.
Teppo, A.-M., von Willebrand, E., Honkanen, E., Ahonen, J. and Gronhagen-Riska, C. (2001). Soluble intercellular adhesion molecule-1 (sicam-1) after kidney transplantation: the origin and role of urinary sicam-1?, Transplantation 71, 1113–1119.
Turley, E. A. (1991). Hyaluronan-binding proteins and receptors, Adv. Drug Deliv. Rev. 7, 257–264.
Turley, E. A., Belch, A. J., Poppema, S. and Pilarski, L. M. (1993). Expression of a receptor for hyaluronan mediated motility on normal and malignant lymphocytes, Blood 81, 446–453.
Von Andrian, U. H., Hasslen, S. R., Nelson, R. D., Erlandeson, S. L. and Butcher, E. C. (1995). A central role for microvillous receptor presentation in leukocyte adhesion under flow, Cell 82, 989–999.
Waller, B. F., Pinkerton, C. A., Orr, C. M., Slack, J. D., Van Tassel, J. W. and Peters, T. (1991). Morphological observations late (greater than 30 days) after clinically successful balloon coronary angioplasty, Circulation 83(Suppl. 2), I28–I41.
Xu, X. M., Chen, Y., Chen, J., Yang, S., Gao, F., Underhill, C. B., Creswell, K. and Zhang, L. (2003). A peptide with three hyaluronan binding motifs inhibits tumor growth and induces apoptosis, Cancer Res. 63, 5685–5690.
Yang, B. H., Yang, B. L., Savani, R. C. and Turley, E. A. (1994). Identification of a common hyaluronan-binding motif in the hyaluronan-binding proteins RHAMM, CD44 and link protein, EMBO J. 13, 286–296.
Author information
Authors and Affiliations
Corresponding author
Additional information
Deceased.
Rights and permissions
About this article
Cite this article
Alam, C.A.S., Seed, M.P., Freemantle, C. et al. The inhibition of neutrophil-endothelial cell adhesion by hyaluronan independent of CD44. Inflammopharmacol 12, 535–550 (2005). https://doi.org/10.1163/156856005774382733
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856005774382733