修回日期: 2004-09-09
接受日期: 2004-09-19
在线出版日期: 2004-10-15
目的: 探讨PFT-a(p-fifty three inhibitor, PFT-a)对热化疗诱导原代培养肠上皮细胞(intestinal epithelial cells, IECs)凋亡及其凋亡相关基因表达的影响.
方法: 原代培养IECs分为正常对照组、热化疗组和PFT-a+热化疗组, 运用顺铂联合温热(43 ℃)处理IECs 30 min, 对比加入不同浓度的PFT-a后, Annexin V-FITC /PI染色, 流式细胞仪检测细胞凋亡. Western blot检测IECs的p53和Bax蛋白表达, RT-PCR检测Bax mRNA的表达.
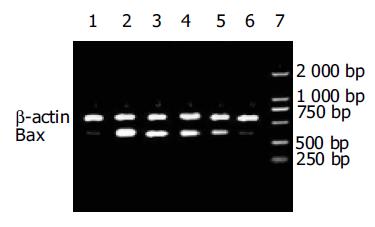
结果: 热化疗导致IECs发生凋亡, Bax蛋白表达明显高于对照组. 10, 20, 30, 40 mmol/L PFT-a作用于顺铂联合温热处理的IECs后, 细胞凋亡率下降且呈剂量依赖性, p53在IECs胞核/胞质表达比例下降, Bax蛋白和mRNA的表达随PFT-a的剂量升高而逐渐下降.
结论: PFT-a对热化疗诱导肠上皮细胞凋亡具有抑制作用, 其机制可能与改变p53核转位和抑制促凋亡基因Bax的表达相关.
引文著录: 张安平, 刘宝华, 张连阳, 王亚旭, 熊仁平, 周萍. p53抑制剂PFT-a对热化疗诱导肠上皮细胞凋亡的保护作用. 世界华人消化杂志 2004; 12(10): 2353-2355
Revised: September 9, 2004
Accepted: September 19, 2004
Published online: October 15, 2004
AIM: To investigate the effect of PFT-a (p53 inhibitor, PFT-a) on apoptosis and apoptosis-associated gene expression in intestinal epithelial cells (IECs) induced by hyperthermic chemotherapy.
METHODS: Primary cultured IECs were divided into control(CON), hyperthermic chemotherapy (HTC) and PFT-a plus hyperthermic chemotherapy group (PFT-a, HTC). IECs in HTC group were treated with hyperthermic (43 ℃) cisplatin (terminal concentration 10 mg/L) for 30 min. IECs in PFT-a, HTC group were treated with different dosages (10, 20, 30, 40 mmol/L) of PFT-a 4 h before treated the same as those in HTC group. The rate of apoptosis was detected using flow cytometry after double staining of annexin V-FITC and PI. The expression of p53 and Bax protein were detected by Western blotting, and the level of bax mRNA expression was detected using semi-quantitative RT-PCR.
RESULTS: PFT-a reduced apoptotic rate of IECs in a dose-dependant manner, from (14.83 ±1.51%) at the concentration of 10mmol/L to (3.75±0.33%) at 40 mmol/L, which were significantly lower than the rate in HCT group(27.03 ±2.07%, P <0.01). The ratio of p53 in the nucleus and cytoplasm was 13.22:1 in HCT group. However, the ratio in PFT-a, HTC group decreased from 0.8:1 at the concentration of 10 mmol/L to 0.4:1 at 40 mmol/L, which were significantly lower than that in HCT group. Hyperthermic chemotherapy upregulated the levels of bax mRNA (128.85±2.38) and protein (14.29 ±0.43) in IECs, whereas PFT-a significantly reduced (P <0.01) those levels in a dose-dependant manner, from 106.98±3.75 (mRNA) and 5.79±0.24 (protein) at the concentration of 10 mmol/L to 4.79±0.31 (mRNA) and 1.36±0.09 (protein) at 40 mmol/L respectively.
CONCLUSION: PFT-a effectively inhibits apoptosis of IECs induced by hyperthermic chemotherapy. The mechanisms may relate to its effect on changing the nuclear translocation of p53and inhibiting the expression of bax gene.
- Citation: Zhang AP, Liu BH, Zhang LY, Wang YX, Xiong RP, Zhou P. An inhibitor of p53 protects intestinal epithelial cells against apoptosis induced by hyperthermic chemotherapy. Shijie Huaren Xiaohua Zazhi 2004; 12(10): 2353-2355
- URL: https://www.wjgnet.com/1009-3079/full/v12/i10/2353.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i10.2353
温热化疗在杀伤肿瘤细胞的同时对增生活跃的IECs产生损伤, 导致抗肿瘤副效应, p53在诱导IECs凋亡中可能起重要作用[1-2]. p53特异性抑制剂PFT-a可以阻断阿霉素、紫杉醇、g射线等诱导的p53激活, 降低正常组织的凋亡[3]. 我们探讨PFT-a抑制顺铂联合温热诱导IECs的p53表达, 以及对IECs损伤的影响及机制.
参照文献[4]进行结肠上皮细胞体外培养. PFT-a为Calbiochem公司产品; 顺铂, MTT, Tripure为Sigma公司产品, DNA Marker DL2000, Ex TaqTM, PCR试剂盒购自Takara公司; M-MLV反转录酶为Promega公司产品; Western Blot发光试剂盒, p53单抗, Bax单抗均购自Santa Cruz公司; Annexin-V-FITC购自BD公司.
将培养的结肠上皮细胞分为3组: (1)正常对照组(control group, Con); (2)热化疗组(hyperthermic chemotherapy group, HTC): 顺铂溶于DMSO中, 终浓度为10 mg/L加入培养板中, 在43 ℃恒温水浴箱中处理30 min后, 用PBS洗细胞2次后, 加入培养基将细胞放入孵箱培养2 h; (3) PFT-a+热化学组(PFT-a plus hyperthermic chemotherapy group, PFT-a+HTC): PFT-a在临用前溶解于DMSO, 以10, 20, 30, 40 mmol/L在热化学处理前4 h加入培养板中, 然后处理同热化学处理组, 在热化学处理完毕后用PBS洗细胞2次, 再重新加入上述PFT-a后将细胞放入孵箱培养2 h. 收集各组细胞, 2 000 r/min, 4 ℃离心5 min后, 冷PBS 重悬, 离心, 弃上清再次重悬离心, 加Annexin-V-FITC 溶液5 mL 和PI 2.5 mL 到100 mL 细胞悬浮液中, 混匀后避光反应10 min, 加150 mL 样品稀释液到样品中, 混匀后上机检测细胞凋亡.提取IECs胞质、胞核和总蛋白, Bradford法测定蛋白浓度后上样, 110 g/L SDS-聚丙稀酰胺凝胶电泳后行半干转膜至PVDF膜上, 封闭液封闭后依次加入p53单抗、Bax单抗(一抗1:400)和辣根过氧化物酶标记的IgG(二抗1:2 500)室温孵育, 再加入化学发光试剂与PVDF膜共孵育后进行自显影.用扫描分析软件系统(Labwork4.0)测量Western blot积分吸光度值(IA), 以正常对照组实际IA值为参照, 其他组别的IA与之相比的相对积分吸光度(RIA)为蛋白的表达量. 另用Tripure试剂提取总RNA, 参照试剂盒取1 mL RNA进行操作. Primer5.0软件进行引物设计. Bax上游引物: 5'- GGATGCGTCCACCAAGAA-3', 下游引物为5'-GCACTCCCGCCACAAAGA-3', 产物长度为386 bp. β-actin为内参照, 上游引物: 5'-CCAAGGCCAACCGCGAGAAGATGAC-3', 下游引物: 5'-AGGGTACATGGTGTGCCGCCAGA-3', 产物长度为587 bp. 引物由上海生工生物公司合成. 条件是94 ℃预变性5 min, 然后94 ℃变性55 s, 50 ℃退火1 min, 72 ℃延伸1 min, 30次循环后, 最后72 ℃ 10 min. 琼脂糖凝胶电泳, 用扫描分析软件系统(Labwork4.0)测量RT-PCR结果的积分吸光度值(IA). 样本与内参照β-actin的IA比值为样本的mRNA相对含量.
统计学处理 所有数据均以平均数±标准差(mean±SD)表示, 采用SPSS10.0统计软件进行分析. 组间比较采用t检验, P <0.01为差异非常显著.
IECs的自然凋亡率在2.88±0.44%, 热化疗损伤后其凋亡率达27.03±2.07%, PFT-a抑制热化疗诱导IECs的凋亡, 且呈剂量依赖性, 凋亡率由14.83±1.51%逐渐下降至3.75±0.33%, 与热化疗组相比凋亡率显著下降(P <0.01).
Western blot分析显示热化疗损伤后p53主要在细胞核表达, 胞核/胞质比为13.22.10 mol/L PFT-a作用后其胞质表达升高, 胞核表达减少, 胞核/胞质比下降至0.80, 40 mmol/L PFT-a作用后这种变化更显著. 与热化疗组相比p53胞核/胞质表达比存在差异显著性(P <0.01)(表1).
腹腔低分化恶性肿瘤常导致肿瘤细胞的腹腔播散, 是术后肿瘤复发的主要原因之一, 而腹腔内温热灌注化疗是腹腔肿瘤播散有效的治疗措施[5-7], 但同时对于增生活跃的正常组织如肠上皮细胞、毛囊细胞和造血干细胞等也产生损伤, 顺铂作为常用的抗肿瘤药物, 可以与DNA结合形成交链, 抑制DNA合成而导致细胞周期停滞和凋亡发生, 而温热可增加细胞对顺铂的摄取, 增强顺铂的抗肿瘤效应[8-10]. p53特异性抑制剂PFT-a是一种小分子化学合成物, 可使经g射线致死剂量照射C57BL和Balb/c两系小鼠的存活达60%, 在照射后7 mo并无新发肿瘤, 认为PFT-a通过抑制p53的激活而能够抑制抗肿瘤治疗的副效应[11]. 我们采用原代结肠上皮细胞为研究对象, 顺铂联合温热处理可导致IECs发生凋亡, p53蛋白向细胞核内聚集, Bcl-2家族的促凋亡基因Bax的蛋白和mRNA表达显著升高.通过运用PFT-a阻断p53由细胞质向胞核转运, 降低Bax的表达, 使IECs的凋亡减少, 提高细胞的生存力. 在研究中发现热化疗损伤后立即去除PFT-a, 则他对肠上皮细胞损伤的保护作用极其微弱, 而PFT-a继续保护2 h, 其保护效应达高峰, 随后下降. Zhang et al[12]发现顺铂常引起耳蜗和前庭感觉毛细胞的损害而导致不可逆转的听力丧失和平衡障碍, 对新生小鼠的前庭和耳蜗进行器官培养, 在顺铂处理的耳蜗和前体中加入PFT-a, 可以使毛细胞的生存率增加且呈剂量依赖性.通过抑制p53的表达, 使顺铂诱导耳蜗和前庭毛细胞的caspase-1, caspase-3活性降低, 明显降低对毛细胞的损害, 为减轻顺铂引起的耳毒性、前庭毒性和神经毒性副作用提供新的方法.Javelaud et al[13]发现PFT-a可以减轻柔红霉素诱导的HCT116p21WAF1-/-细胞的凋亡, PFT-a抑制p53而使Bcl-2的表达水平升高, Bax的表达水平下降, 恢复Bax/Bcl-2比率, 其作用与p21WAF1反义寡核苷酸相似. Liu et al报道了PFT-a对阿霉素诱导心肌细胞凋亡和心功能损害的保护效应. 阿霉素上调Bax和MDM2mRNA水平, 通过p53在丝氨酸15的磷酸化水平提高上调p53蛋白水平, 诱导心肌细胞凋亡导致阿霉素的心脏毒性、超微结构改变和心功能障碍.在给予阿霉素前30 min和给药后3 h使用PFT-a, 则心肌细胞Bax和MDM2mRNA水平下降, 实验表明PFT-a可有效抑制阿霉素诱导的心肌细胞凋亡, 减轻阿霉素抗肿瘤治疗中对心脏的损伤.
我们发现PFT-a抑制热化疗诱导结肠上皮细胞凋亡, 降低对细胞的损伤, 为以后通过抑制p53而减轻抗肿瘤副效应提供了新的思路, 但是PFT-a发挥保护作用的机制还有待深入研究.
| 1. | van der Vange N, van Goethem AR, Zoetmulder FA, Kaag MM, van de Vaart PJ, ten Bokkel Huinink WW, Beijnen JH. Extensive cytoreductive surgery combined with intra-operative intraperitoneal perfusion with cisplatin under hyperthermic conditions (OVHIPEC) in patients with recurrent ovarian cancer: a feasibility pilot. Eur J Surg Oncol. 2000;26:663-668. [PubMed] [DOI] |
| 2. | Shen P, Levine EA, Hall J, Case D, Russell G, Fleming R, McQuellon R, Geisinger KR, Loggie BW. Factors predicting survival after intraperitoneal hyperthermic chemotherapy with mitomycin C after cytoreductive surgery for patients with peritoneal carcinomatosis. Arch Surg. 2003;138:26-33. [PubMed] [DOI] |
| 3. | Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733-1737. [PubMed] [DOI] |
| 4. | Grossmann J, Walther K, Artinger M, Kiessling S, Steinkamp M, Schmautz WK, Stadler F, Bataille F, Schultz M, Schölmerich J. Progress on isolation and short-term ex-vivo culture of highly purified non-apoptotic human intestinal epithelial cells (IEC). Eur J Cell Biol. 2003;82:262-270. [PubMed] [DOI] |
| 5. | Xu DZ, Zhan YQ, Sun XW, Cao SM, Geng QR. Meta-analysis of intraperitoneal chemotherapy for gastric cancer. World J Gastroenterol. 2004;10:2727-2730. [PubMed] [DOI] |
| 6. | Hu JK, Chen ZX, Zhou ZG, Zhang B, Tian J, Chen JP, Wang L, Wang CH, Chen HY, Li YP. Intravenous chemotherapy for resected gastric cancer: meta-analysis of randomized controlled trials. World J Gastroenterol. 2002;8:1023-1028. [PubMed] [DOI] |
| 7. | Ye M, Pan HM, Wang HY, Lou F, Jin W, Zheng Y, Wu JM. Catheterization-associated complications of intraperitoneal chemotherapy in advanced gastric cancer. World J Gastroenterol. 2004;10:1372-1374. [PubMed] |
| 8. | Ryu KS, Kim JH, Ko HS, Kim JW, Ahn WS, Park YG, Kim SJ, Lee JM. Effects of intraperitoneal hyperthermic chemotherapy in ovarian cancer. Gynecol Oncol. 2004;94:325-332. [PubMed] [DOI] |
| 9. | Piso P, Dahlke MH, Loss M, Schlitt HJ. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from ovarian cancer. World J Surg Oncol. 2004;2:21. [PubMed] [DOI] |
| 10. | Hall JJ, Loggie BW, Shen P, Beamer S, Douglas Case L, McQuellon R, Geisinger KR, Levine EA. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg. 2004;8:454-463. [PubMed] [DOI] |
| 11. | Komarova EA, Gudkov AV. Suppression of p53: a new approach to overcome side effects of antitumor therapy. Biochemistry (Mosc). 2000;65:41-48. [PubMed] |
| 12. | Zhang M, Liu W, Ding D, Salvi R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience. 2003;120:191-205. [PubMed] [DOI] |
| 13. | Javelaud D, Besancon F. Inactivation of p21WAF1 sensitizes cells to apoptosis via an increase of both p14ARF and p53 levels and an alteration of the Bax/Bcl-2 ratio. J Biol Chem. 2002;277:37949-37954. [PubMed] [DOI] |