修回日期: 2002-07-20
接受日期: 2002-07-31
在线出版日期: 2003-05-15
研究胃癌、癌前病变(异型增生、肠化生、萎缩性胃炎)细胞增生凋亡及其调节基因表达, 探讨胃癌多阶段发生.
采用病理组织学对24例胃癌、14例癌旁异型增生、24例远离癌灶的慢性胃炎和32例肠化生胃黏膜进行分类、分期、分度, Ki67, P53及Bcl-2免疫组化染色, TUNEL(ISEL)技术检测细胞凋亡. 计算各种病变的Ki67增生指数和凋亡指数、凋亡增生比、凋亡强度, 和P53, Bcl-2的阳性例数和百分比.
胃癌、异型增生、肠化生和萎缩性胃炎的增生指数分别是12.4±6.8%, 6.4±4.2%, 6.6±3.4%和3.8±2.9%, 浅表性胃炎与各组均有非常显著差异(P<0.01).凋亡指数分别是2.4±1.4%, 2.6±1.6%、3.1±1.2%和3.8±2.0%; 凋亡强度分别是0.3, 0.6, 079和1.3. 以萎缩性胃炎最高, 与其他各组间均有非常显著差异(P<0.01), 中、重度异型增生和胃癌相互间无显著性差异(P>0.05). 重度异型增生和早期胃癌的凋亡强度是浅表性胃炎的1/3, 进展期胃癌仅为其1/5. P53蛋白在胃癌、异型增生、肠化生、萎缩性胃炎分别是25.0%, 14.3%, 3.1%和0%. Bcl-2的表达分别是58.3%, 42.8%, 9.4%和7.1%, 在胃癌和异型增生P53蛋白和Bcl-2表达相互间存在显著相关性(P<0.05).
P53和Bcl-2基因不仅在胃癌表达, 在癌前病变异型增生、肠化生也有部分表达, 萎缩性胃炎不具有上述特点.
引文著录: 潘传敬, 刘宽宇. 胃癌增生凋亡与调节基因的表达. 世界华人消化杂志 2003; 11(5): 526-530
Revised: July 20, 2002
Accepted: July 31, 2002
Published online: May 15, 2003
To study proliferation and apoptosis in gastric carcinoma (GC) and precancerous lesions and expression of their regulating genes.
Total 24 cases of GC, 14 dysplasia (DP), 24 chronic gastritis (10 superfical gastritis, SG, 14 atrophic gastritis, AG) and 32 intestinal metaplasia (IM) were studied by histopathological, immunohistochemical techniques with Ki67, P53 and Bcl-2 antibodies, and by TUNEL for apoptosis. Proliferation index (PI), apoptosis index (AI), apoptosis/ proliferation ratio, apoptosis degree and positive percent of P53 and Bcl-2 were counted.
The PI of GC, DP, IM, AG were 12.4±6.8%, 6.4±4.2%, 6.6±3.4%, 3.8±2.9% respectively. There was a significant difference between SG and GC, DP, IM, and AG (P<0.01). The AI of GC, DP, IM, and AG was 2.4±1.4%, 2.6±1.6%, 3.1±1.2% and 3.8±2.0% respectively. The apoptosis degree of AG was the highest, and significantly higher than that of GC, DP, and IM (P<0.01). There was no significantly different among GC, moderate and severe DP (P>0.05). Mucosa in AG showed excessive apoptosis, however the apoptosis degree in severe DP and early gastric carcinoma was about 1/3 of SG, and in advanced GC was its 1/5. The positive percentages of P53 in GC, DP, IM and AG were 25.0%, 14.3%, 3.1% and 0% respectively. The positive rate of Bcl-2 was 58.3%, 42.8%, 9.4% and 7.1% respectively. There was a significant correlation beween P53 and Bcl-2.
Mutated P53 gene and Bcl-2 apoptosis regulating gene not only express in GC, also partly express in DP, IM, and in those precancerous lesions in which gastric carcinogenesis may have been initialized. The imbalance between proliferation and apoptosis is manifestation of the gene mutations on the cytodynamics. The DP and IM may be the different stages in gastric carcinogenesis.
- Citation: Pan CJ, Liu KY. Proliferation/apoptosis and expression of P53 and Bcl-2 in gastric carcinoma. Shijie Huaren Xiaohua Zazhi 2003; 11(5): 526-530
- URL: https://www.wjgnet.com/1009-3079/full/v11/i5/526.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i5.526
胃癌的发生发展是一个多基因突变累积, 逐渐演变多步骤发生的复杂过程[1-13]. 萎缩性胃炎、肠化生和异型增生等癌前病变与胃癌的发生关系密切[14-40]. 肠化生微卫星不稳定性的阳性率和分布、细胞凋亡障碍等都与胃癌的表现相似, 显示肠化生等癌前病变在细胞动力学和癌发生早期分子生物学与胃癌有内在关系[16,22]. 为此, 我们观察了胃癌、癌旁异型增生、肠化生、萎缩性胃炎等癌前病变的细胞增生与凋亡改变, 以及他们对抑癌基因P53和凋亡调节基因Bcl-2表达, 探讨他们的内在关系.
本院2000-04/2001-09手术切除胃癌24例, 男17例, 女7例, 年龄35-76(平均49)岁, 每例在胃癌、癌旁和远离癌灶的胃黏膜组织各取3块. 另选同期胃镜活检病理诊断为肠化生的32例, 男24例, 女8例, 年龄31-72(平均45)岁, 每例在胃窦钳取3-4块黏膜组织. 胃癌中早期胃癌5例, 进展期19例, 其中11例有淋巴结转移. 癌旁胃黏膜中有14例不同程度的异型增生, 其中轻度3例, 中度6例, 重度5例, 10例伴有肠化生. 远离癌灶的胃黏膜呈慢性胃炎改变, 浅表性10例, 萎缩性14例. 32例肠化生均在中度以上广泛肠化.
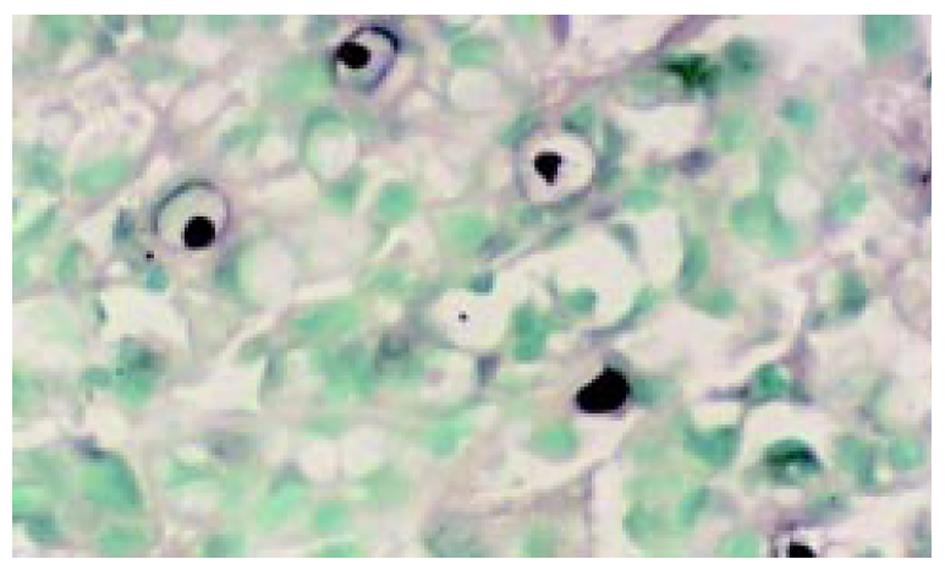
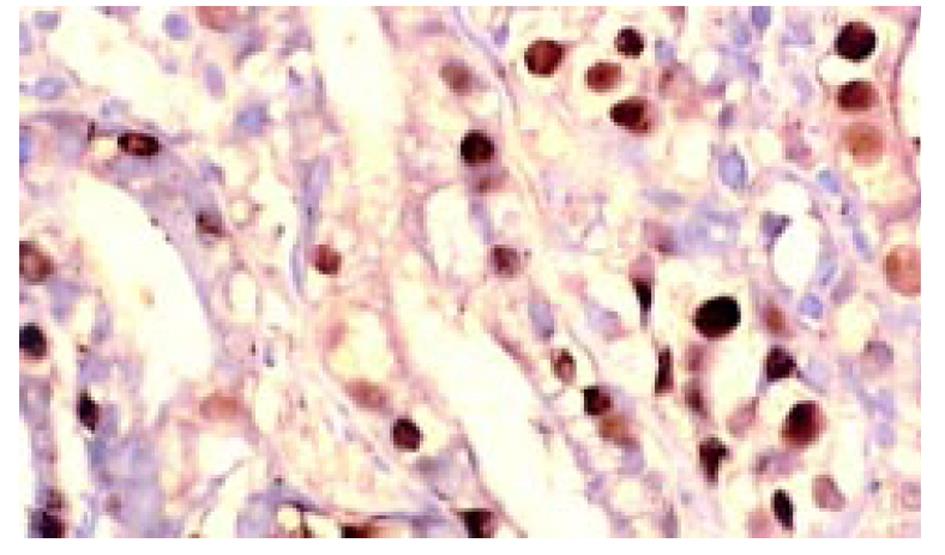
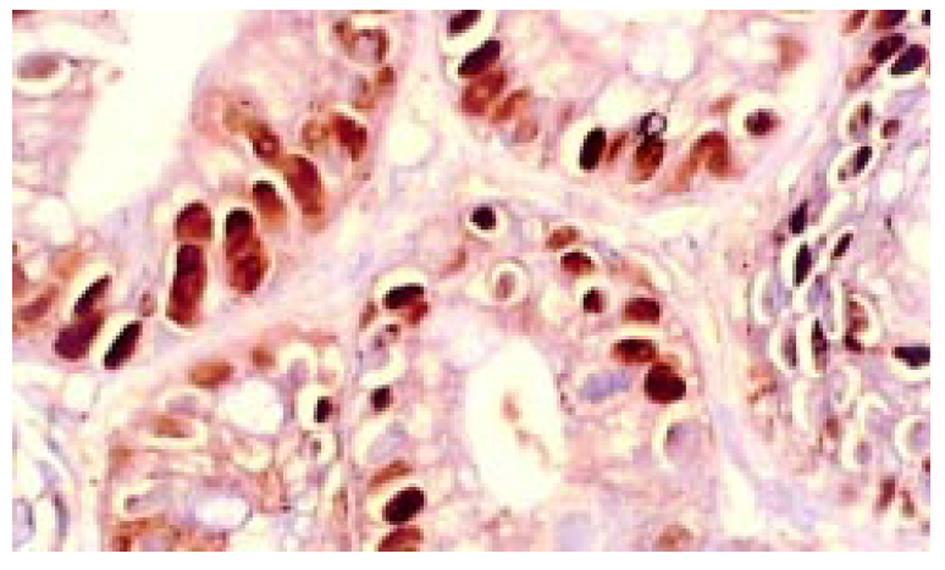
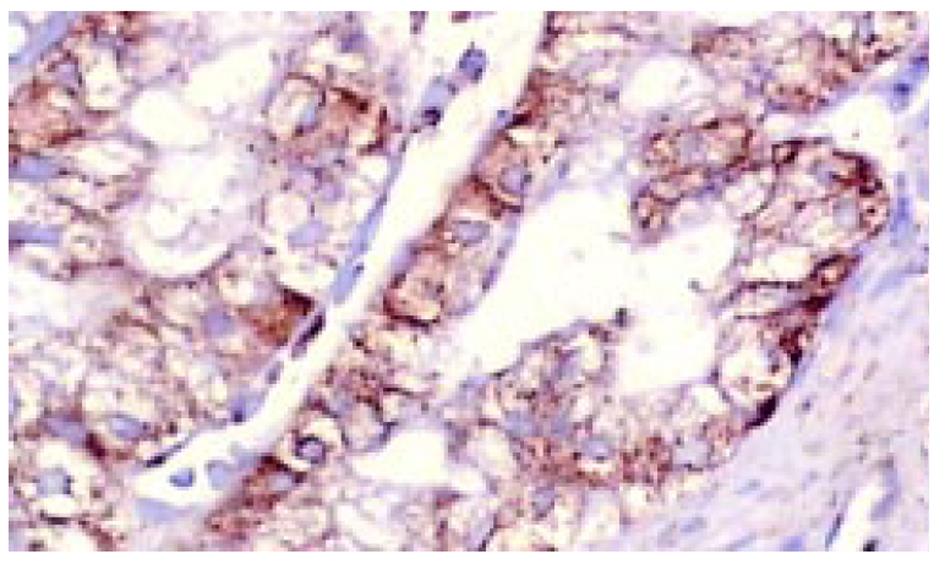
所有组织用中性甲醛固定, 常规脱水, 石蜡包埋, 制备5 μm连续切片6张, 分别作HE染色组织学诊断, 作Ki67、P53蛋白、Bcl-2免疫组织化学染色. TUNEL(ISEL)技术检测细胞凋亡. ISEL技术用Boehringer产品, 为石蜡切片脱蜡至水干燥; 10 mg/L蛋白酶K(Gibco)消化, 37 ℃, 1 h, 漂洗2次, 擦干组织周围水分, 用DAKO笔划圈; 末端标记: 10 mmol/L t ris盐酸缓冲液(PH7.63)内含50 mmol/L氯化镁10 mmol/L α-巯基嘌呤醇, 0.5 g/L血清清蛋白及20 ku/L DNA多聚酶 0.01 mmol/L dATP, dCTP, dGTP和0.01 mmol/L地高辛标记的dUTP, 每片切片滴标记混合液50 μL, 37 ℃, 1 h, 漂洗3次, 滴碱性磷酸酶标记的羊抗地高辛多克隆抗体50 μL, 37 ℃, 30 min, NBT/BCIP显色, 室温10 min, 流水洗, 用20 g/L甲基绿复染, 加拿大胶封片观察. ISEL标记细胞凋亡阳性清晰地显示在洁净的背景上, 核呈兰黑色, 轮廓清楚, 致密成团块状, 有的呈新月形, 有空晕, 阴性胞核作淡绿色(图1). Ki67免疫组化染色阳性集中在核呈棕黄色, 致密均匀一致(图2). P53染色阳性分布于胞核呈棕黄色至深棕黄色(图3). Bcl-2染色阳性分布于胞质, 呈棕黄色颗粒状(图4). 对Ki67染色和ISEL进行定量计数, 选染色典型的连续5个高倍视野计数1000个胃癌细胞或黏膜上皮细胞和其中的阳性细胞. 计算每100个细胞中的阳性数, 所得Ki67参数为增生指数(PI), ISEL参数为凋亡指数(AI). 用各组PI, AI求凋亡增生比; 以浅表性胃炎为基数1, 求各组的凋亡强度. P53和Bcl-2的阳性细胞数在10%以上者为阳性例数, 按例计数. 对各组检测数据进行统计学处理, 进行显著性检验.
依浅表性胃炎、萎缩性胃炎、肠化生、异型增生、胃癌的顺序随疾病的进展, Ki67检测的增生指数逐渐升高. 以进展期胃癌为最高, 达13.0±7.4%, 浅表性胃炎与萎缩性胃炎、肠化生、异型增生、胃癌均有非常显著的差异(P<0.01), 他们的细胞增生显著加速. 凋亡指数以萎缩性胃炎为最高3.8±2.0%, 与各组均有非常显著差异(P<0.01), 其余各组凋亡指数均较低, 中、重度异型增生和胃癌相互间无显著性差异(P>0.05, 表1). 凋亡增生比和凋亡强度以萎缩性胃炎凋亡强度最大, 是浅表性胃炎的1.34倍; 其余肠化生、异型增生和胃癌均比浅表性胃炎低.重度异型增生和早期胃癌是浅表性胃炎的1/3; 进展期胃癌仅为其1/5. 胃癌和癌前病变都出现明显的凋亡障碍(表1).
| 病变类型 | n | 增生指数 | 凋亡指数 | AI/AP | 凋亡强度 |
| 胃癌 | 24 | 12.4±6.8b | 2.4±1.4a | 0.2 | 0.3 |
| 早期 | 5 | 11.9±6.1 | 2.8±0.9 | 0.2 | 0.3 |
| 进展期 | 19 | 13.0±7.4 | 2.1±1.7 | 0.2 | 0.2 |
| 异型增生 | 14 | 6.4±4.3b | 2.6±1.6a | 0.4 | 0.6 |
| 轻度 | 3 | 4.7±2.0 | 2.9±1.2 | 0.6 | 0.9 |
| 中度 | 6 | 5.7±3.2 | 2.8±1.1 | 0.5 | 0.7 |
| 重度 | 5 | 9.5±5.0 | 2.1±0.9 | 0.2 | 0.3 |
| 肠化生 | 32 | 6.6±3.4b | 3.1±1.2a | 0.5 | 0.7 |
| 慢性胃炎 | 24 | 3.8±2.9 | 3.4±1.3 | 0.9 | 1.2 |
| 浅表性 | 10 | 2.3±1.5 | 1.6±0.7 | 0.7 | 1.0 |
| 萎缩性 | 14 | 4.1±2.8 | 3.8±2.0 | 0.9 | 1.3 |
P53蛋白在胃癌、异型增生、肠化生和萎缩性胃炎中表达率分别是25.0%, 14.3%, 3.1%和0%. 肠化生与异型增生、胃癌之间有非常显著差异(P<0.01), 异型增生和胃癌之间有显著性差异(P<0.05). Bcl-2在胃癌、异型增生、肠化生和萎缩性胃炎中的表达分别是58.3%, 42.8%, 9.4%和7.1%. 肠化生与异型增生、胃癌之间均有非常显著差异(P<0.01), 异型增生与胃癌之间有显著性差异(P<0.05, 表2).
胃癌是严重危害人类健康最常见的癌症之一[1], 占我国癌发病和死亡率之首[41]. 且有逐年上升趋势. 对他的研究已深入到多种基因突变和蛋白分子表达[1,4-7,9-13,16]. 有的通过反义基因治疗达到抑制肿瘤增生生长的作用[1,3,8]. 利用胃癌和癌前病变细胞动力学和细胞生物学表现规律[15,17,18], 探讨及早发现病理组织学的微小变化, 是胃癌早防早治的关键, 也是科学研究和临床诊治的良好结合点. 我们将二者结合对照分析, 发现浅表性胃炎、萎缩性胃炎、肠化生、异型增生随病变加重, 细胞增生指数逐渐升高. 在发展的不同阶段, 细胞增生指数有显著差异. 胃癌的增生指数最高. 细胞凋亡指数显示: 除萎缩性胃炎凋亡明显增加外, 肠化生、异型增生凋亡指数较低, 有的与胃癌相近. 出现较高水平的增生和低水平的凋亡. 特别是他们的凋亡增生比和凋亡强度更清楚地显示增生和凋亡已明显失去平衡. 这大大增加了病变细胞的不稳定性, 促进病变细胞基因突变, 导致胃癌发生发展. 在癌前病变出现过高水平的增生和凋亡障碍会引起细胞聚积, 可能是形成微小胃癌和早期胃癌的重要细胞动力学基础. 本组5例早期胃癌, 有3例都在肠化生、异型增生背景下出现多灶性微小癌, 而主要癌灶出现黏膜内浸润. 这是对胃癌早期发生细胞动力学的佐证. 进展期胃癌过度的细胞增生和严重的凋亡障碍是肿瘤迅速生长和广泛扩散的病理学基础.表现为细胞核分裂、增生极为活跃和去分化、异质性分化、出现异型核、巨核癌细胞等中晚期癌的鲜明特征.
免疫组化染色检出的P53蛋白异常表达常代表突变型P53基因, 已丧失了抑制肿瘤生长, 反而具有致癌作用, 成为癌基因. 最常见于胃癌[1,11,19,20,29,32]. 我们发现P53蛋白不仅表达在胃癌(25.0%), 癌前病变也有部分表达. 除萎缩性胃炎无表达外, 肠化生表达3.1%, 异型增生14.3%. 提示肠化生、异型增生有抑癌基因突变存在, 可能是引起他们增生加快和凋亡受阻的重要原因之一. Bcl-2基因抑制细胞凋亡, 延长细胞寿命, 促进肿瘤的发生、发展. 我们发现除胃癌Bcl-2有高达58.3%外, 萎缩性胃炎、肠化生、异型增生也有不同程度表达, 异型增生达42.8%, 提示Bcl-2表达与胃癌的发生密切相关, 在癌前病变即有Bcl-2基因的广泛参与. 在这个过程中由于凋亡抑制基因的多重作用, 少数永生化细胞已经产生, 他们实际上是一种癌前细胞. 检测同时发现P53蛋白表达和Bcl-2蛋白表达之间存在显著相关性(P<0.05). 与以往研究结果一致[11,20,22,26,32], 在胃癌发生过程中P53基因突变, 其蛋白产物增加, 细胞增生加快, 凋亡受阻, 使高度增生的幼稚细胞聚积. 相应凋亡抑制基因Bcl-2表达也增加, 又使增生的幼稚细胞生存延长, 处于不稳定状态, 还可致该细胞群P53基因突变加剧, 引起细胞恶性增生、癌形成. 二者之间存在着既独立、又协同促进胃癌发生发展的作用. 胃癌很高的增生和严重的凋亡受阻, 导致分化幼稚不成熟的肿瘤性细胞聚积, 成为病理组织学诊断胃癌的形态依据, 相应得到P53基因突变和Bcl-2凋亡抑制基因表达一致的佐证和支持. P53基因突变和Bcl-2凋亡抑制基因表达在癌前病变肠化生和异型增生也有部分表达. 这些病变已有少数永生化细胞产生. 从基因水平分析这些病变癌发生的过程已经启动, 细胞增生和凋亡的严重失衡是其细胞动力学上的表现. 随着癌发生过程的进展, 病变程度加重其基因表达和细胞动力学失衡越明显. 永生化的癌前细胞演变成癌细胞, 相应在病理形态上也出现早期癌特征[1,14,15]. 提示肠化生、异型增生的不同程度可能是胃癌发生多步骤中不同阶段的表现. 萎缩性胃炎与肠化生、异型增生有所不同, 虽有较高水平的细胞增生, 也伴有高水平的凋亡, 其结果是黏膜上皮细胞减少[14,15], 不存在凋亡障碍和幼稚细胞聚积, 更重要的是无明显基因突变表达, 缺乏癌发生发展的基础, 不具有癌前病变的特点. 因此萎缩性胃炎不伴有广泛肠化生或异型增生者, 不宜归于癌前病变的范围.
| 1. | Wang GT. Progress in studies of mechanism of gastric precancerous lesions, carcinogenesis and their reversion. ShijieHuarenXiaohuaZazhi. 2000;8:1-4. |
| 2. | Wang RQ, Fang DC, Liu WW. MUC 2 gene expression in gastric cancer and preneoplastic lesion tissues. ShijieHuarenXiaohuaZazhi. 2000;8:285-288. |
| 3. | Du JJ, Dou KF, Cao YX, Wang ZH, Wang WZ, Gao ZQ. CAll, a down-regulated gene in gastric cancer: a functional study. ShijieHuarenXiaohuaZazhi. 2002;10:525-529. |
| 4. | Zhang YM, Deng CS, Zhu YQ, Mao YR, Zhang K, Yang YP. Correlation between expression of hypoxia-inducible factor-1αmRNA and angiogenesis in gastric adenocarcinoma. ShijieHuarenXiaohuaZazhi. 2002;10:633-637. |
| 5. | He XX, Wang JL, Wu JL, Yuan SY, Ai L. Telomerase expression, Hp infection and gastric mucosal carcinogenesis. ShijieHuarenXiaohuaZazhi. 2000;8:505-508. |
| 6. | He XX, Wang JL, Wu JL, Yuan SY, Ai L. Telomere, cellular DNA content and gastric mucosal carcinogenesis. ShijieHuarenXiaohuaZazhi. 2000;8:509-512. |
| 7. | Wang W, Luo HS, Yu BP. Expression of human telomerase reverse transcriptase gene and c-myc protein in gastric carcinogenesis. ShijieHuarenXiaohuaZazhi. 2002;10:258-261. |
| 8. | Yang SM, Fang DC, Yang JL, Luo YH, Lu R, Liu WW. Effect of antisense gene to human telomerase reverse transcriptase on telomerase activity and expression of apoptosis-associated gene. ShijieHuarenXiaohuaZazhi. 2002;10:149-152. |
| 9. | Liu HF, Liu WW, Fang DC, Yang SM, Wang RQ. Bax gene expression and its relationship with apoptosis in human gastric carcinoma and precancerous lesions. ShijieHuarenXiaohuaZazhi. 2000;8:665-668. |
| 10. | Zheng ZH, Sun XJ, Qiu GR, Liu YH, Wang MX, Sun KL. E-cadherin gene mutation in precancerous condition early and advanced stages of gastric cancer. ShijieHuarenXiaohuaZazhi. 2002;10:153-156. |
| 11. | Wang DX, Fang DC, Liu WW. Study on alteration of multiple genes in intestinal metaplasia, atypical hyperplaisa and gastric cancer. ShijieHuarenXiaohuaZazhi. 2000;8:855-859. |
| 12. | Luo ZB, Luo YH, Lu R, Jin HY, Zhang PB, Xu CP. Immunohistochemical study on dendritic cells in gastric mucosa of patients with gastric cancer and precancerous lesions. ShijieHuarenXiaohuaZazhi. 2000;8:400-402. |
| 13. | Guo YQ, Zhu ZH, Li JF. Flow cytometric analysis of apoptosis and proliferation in gastric cancer and precancerous lesion. ShijieHuarenXiaohuaZazhi. 2000;8:983-987. |
| 14. | Pan CJ, Zhong P, Huang XR, Liu KY, Wang SX. Study on the correlation between proliferation and apoptosis in atrophy and intestinal metaplasia of gastric mucosa. ShijieHuarenXiaohuaZazhi. 2000;8:143-146. |
| 15. | Lu SY, Pan XZ, Peng XW, Shi ZL, Lin L, Chen MH. Effect of Hp infection on gastric epithelial cell kinetics in stomach diseases. ShijieHuarenXiaohuaZazhi. 2000;8:386-388. |
| 16. | Fang DC, Zhou XD, Luo YH, Wang DX, Lu R, Yang SM, Liu WW. Microsatellite instability and loss of heterozygosity of suppressor gene in gastric cancer. ShijieHuarenXiaohuaZazhi. 1999;7:479-481. |
| 17. | Mi JQ, Zhang ZH, Shen MC. Significancer of CD44v6 protein expression in gastric carcinoma and precancerous lesions. ShijieHuarenXiaohuaZazhi. 2000;8:156-158. |
| 18. | Liang WJ, Ma YJ, Zhang WD, Chen YP. Relationship between classification of spleen syndromes of patients with chronic gastric diseases and gastric carcinoma and cyclin E expression. ShijieHuarenXiaohuaZazhi. 2000;8:513-515. |
| 19. | Wang DX, Fang DC, Li W, Du QX, Liu WW. A study on relationship between infection of Helicobacter pylori and inactivation of antionogenes in cancer and pre-cancerous lession. ShijieHuarenXiaohuaZazhi. 2001;9:984-987. |
| 20. | Cheng SY, Wang JY, Ji Y, Zhang XD, Zhu CW. Effects of Helicobacter pylori and protein kinase C on gene mutation in gastric cancer and precancerous lesions. ShijieHuarenXiaohuaZazhi. 2001;9:302-307. |
| 21. | Bajtai A, Hidvegi J. The role of gastric mucosal dysplasia in the development of gastric carcinoma. PatholOncol Res. 1998;4:297-300. [DOI] |
| 22. | Leung WK, Kim JJ, Ki JG, Graham DR, Sepulveda AY. Microsatellite instability in gastric intestinal metaplasia in patients with and without gastric cancer. Am J Pathol. 2000;156:537-542. [DOI] |
| 23. | Kim YH, Kim NG, Lim JG, Park C, Kim H. Chromosomal alterations in paired gastric adenomas and carcinomas. Am J Pathol. 2001;158:655-662. [DOI] |
| 24. | Ruol A, Parenti A, Zaninotto G, Merigliano S, Costantini M, Cagol M, Alfieri R, Bonavina L, Peracchia A, Ancona E. Intestinal metaplasia is the probable common precursor of adenocarcinoma in barrett esophagus and adenocarcinoma of the gastric cardia. Cancer. 2000;88:2520-2528. [DOI] |
| 25. | Liu HF, Liu WW, Fang DC. Study of the relationship between apoptosis and proliferation in gastric carcinoma and its precancerous lesion. ShijieHuarenXiaohuaZazhi. 1999;7:649-651. |
| 26. | Zhou Y, Gao SS, Li YX, Fan ZM, Zhao X, Qi YJ, Wei JP, Zou JX, Liu G, Jiao LH. Tumor suppressor gene P16 and Rb expression in gastric cardia precancerous lesions from subjects at a high incidence area in northern China. World J Gastroentrol. 2002;8:423-425. [DOI] |
| 27. | Lin CK, Lai KH, Lo GH, Cheng JS, Hsu PI, Mok KT, Tseng HH. Cathepsin E and subtypes of intestinal metaplasia in carcinogenesis of the human stomach. ZhonghuaYixueZazhi (Taipei). 2001;64:331-336. |
| 28. | Yang SM, Fang DC, Luo YH, Lu R, Battle PD, Liu WW. Alterations of telomerase activity and terminal restriction fragment in gastric cancer and its premalignant lesions. J GastroenterolHepatol. 2001;16:876-882. [PubMed] [DOI] |
| 29. | Xu A, Li S, Liu J. Correlation between apoptosis and proliferation in gastric pre-carcinoma. ZhonghuaYixueZazhi. 1999;79:185-186. |
| 30. | Mingchao , Devereux TR, Stockton P, Sun K, Sills RC, Clayton N, Portier M, Flake G. Loss of E-cadherin expression in gastric intestinal metaplasia and later stage p53 altered expression in gastric carcinogenesis. Exp Toxicol Pathol. 2001;53:237-246. [PubMed] [DOI] |
| 31. | Wang D, Fang D, Liu W. Induction of intestinal mataplasia in stomach of dogs and expression of tumor-related proteins in animal gastric mucosa lesions. Chin Med J. 2000;113:336-339. |
| 32. | Lu W, Chen L, Dong H. Helicobacter pylori infection and expression of PCNA, p53, c-erbB-2 in carcinoma and precancerous lesions of the stomach. ZhonghuaZhongliuZazhi. 1999;21:125-127. |
| 33. | Wang J, Chi DS, Kalin GB, Sosinski C, Miller LE, Burja I, Thomas E. Helicobacter pylori infection and oncogene expressions in gastric carcinoma and precursor lesions. Dig Dis Sci. 2002;47:7-13. [DOI] |
| 34. | Conchillo JM, Houben G, de Bruine A, Stockbrugger R. Is type III intestinal metaplasia an obligatory precancerous lesion in intestinal-type gastric carcinoma? Eur J Cancer Prev. 2001;10:307-312. [PubMed] [DOI] |
| 35. | Lu SY, Pan XZ, Pen XW, Shi ZL. Effect of Hp infection on gastric epithelial cell kinetics in stomach diseases. ShijieHuarenXiaohuaZazhi. 1999;7:760-762. |
| 36. | Ushijima T, Yamamoto M, Suzui M, Kuramoto T, Yoshida Y, Nomoto T, Tatematsu M, Sugimura T, Nagao M. Chromosomal mapping of genes controlling development, histological grade, depth of invasion, and size of rat stomach carcinomas. Cancer Res. 2000;60:1092-1096. [PubMed] |
| 37. | Ge CQ, Wang YP, Liu GY, Ma SW, Ding G, Li JC. Study on Helicobacter pylori infection and p53, c-erbB-2 gene expression in carcinogenesis of gastric mucosa. ShijieHuarenXiaohuaZazhi. 1999;7:313-315. |
| 38. | Qing LJ. In situ hybridization of p53 tumor suppressor gene in human gastric precancerous lesions and gastric cancer. ShijieHuarenXiaohuaZazhi. 1999;7:494-497. |
| 39. | Liu HF, Liu WW, Fang DC, Men YP, Wang ZH. Apoptosis and its relationship with Fas ligand expression in gastric carcinoma and its precancerous lesion. ShijieHuarenXiaohuaZazhi. 1999;7:561-563. |
| 40. | Yang L, Wang YP, Wu DY, Zhang SM, Li JY, Zhang YC, Xin Y. Pathological behaviors and molecular mechanisms of signet-ring cell carcinoma and mucinous adenocarcinoma of stomach: a comparative study. ShijieHuarenXiaohuaZazhi. 2002;10:516-524. |
| 41. | Deng DJ, Z E. Overview on recent studies of gastric carcinogenesis :human exposure of N-nitrosamides. ShijieHuarenXiaohuaZazhi. 2000;8:250-252. |