Abstract
Neurodegenerative diseases (NDD) are progressive degenerative disorders of the neurological system with significant social impact worldwide. Their detection at the initial stage is necessary to provide proper therapeutic interventions. Biosensors have emerged as one of the next-generation tools for detecting and monitoring physiochemical changes associated with neurological disorders. This article discusses the current status and challenges of different state-of-the-art sensors which can detect NDD biomarkers. A brief overview of developing advanced biosensors with the help of nanotechnology integration, mainly polymer-based functional nanomaterials, has been mentioned as the prospect of these biosensors for NDD detection and management.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
The gradual loss of neurons' structure or function is commonly termed the neurodegeneration process. This cellular and network-level damage of neurons impairs normal brain functions and gives rise to different neurodegenerative disorders (NDD). Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), multiple sclerosis (MS), etc., are the most common examples of neurodegenerative disorders. 1 At the molecular level, neuroscientists have pointed out aberrant protein assembly or cell apoptosis in the different brain regions, followed by oxidative stress and neuroinflammation as the two critical factors for NDD. For, e.g. in AD, there is a deposition of amyloid-beta peptide (Aβ), leading to the formation of senile plaques in the brain areas associated with cognition. On the other hand, there is a loss of dopaminergic neurons in the substantia nigra in the case of PD patients. 2,3 As per the 2020 report by the Lancet Commission, 115 million individuals will suffer from NDD by the year 2050 due to an increase in the life expectancy of humans. 4
Neurotransmitters are the chemical messengers of the brain which establish the functional connection between the brain and body to regulate behavioural, cardiovascular, renal, and hormonal functions. Any slight change in the neurotransmitter level can lead to mental disorders, physiological disorders and NDD. 5 Due to the presence of the Hypothalamic-Pituitary-Adrenal (HPA) axis and the gut-brain axis, people with NDD have abnormally high hormone and metal-ion levels relative to the general population, as well as volatile chemical concentrations in exhaled breath. 6 All these undesired changes lead to dementia, disorientation, language comprehension problems, communication problems, depression, tremors, muscle rigidity, body imbalance, urinary problems or eating disorders in the patients. 1 Therefore, detecting any change in the concentration of these different biomarkers or biomolecules at an early stage can help medical practitioners with proper and prompt treatment. Though the research on cellular and animal models has shed light on the pathophysiology of NDD and helped us to design suitable therapeutic interventions, NDD is still considered to be incurable due to their detections at a very late age (during which most of the neurons die), complex nature of the disorders, multi-factorial causative agents and inaccessibility of various treatment drugs due to the presence of blood-brain-barrier. 7
For the proper treatment of NDD or to design a suitable therapeutic intervention or management protocol, one should be able to detect the biochemical and cellular changes in the brain at a very early stage. 8 At present, genetic testing, clinical diagnostic tests, brain imaging, and biochemical tests are the four primary techniques for the early identification of NDD. Genetic testing involves the sequencing of genes to pinpoint any change in the genome, chromosome or functional protein that might be linked to a particular NDD. However, the hefty expenses connected with this strategy could be a turnoff for many patients. 9 Clinical diagnosis involves identifying an illness or damage based on the signs and symptoms the patient is exhibiting as well as their medical history and physical examination in the clinical setup. However, the requirement for further testing, such as blood tests or biopsies after a clinical diagnosis and the subjective nature of the diagnosis method are two demerits. 10–12 Therefore, these days brain imaging with advanced techniques like positron emission tomography (PET), magnetic resonance imaging (MRI), functional magnetic resonance imaging (fMRI), single photon emission computed tomography (SPECT), and electron encephalography (EEG) has grown in popularity in the early assessment of NDD. However, the high cost of equipment, unavailability of early scanning slots and non-suitability for patients with metallic devices (like pacemakers) are some of the significant disadvantages of brain imaging. 13,14 On the other hand, biochemical tests include sensing the change in the concentration of different proteins, RNAs, metabolic products, and small molecules in the blood and cerebral fluid (CSF) to focus more precisely on the biochemical abnormalities in NDD. Due to its capacity to identify biomarker concentrations in the blood and CSF, as well as its cheaper cost, greater stability, higher sensitivity, and ease of downsizing, electrochemical sensors have received a lot of attention in recent years. 15,16
Advanced sensors for the detection of neurodegenerative disorders
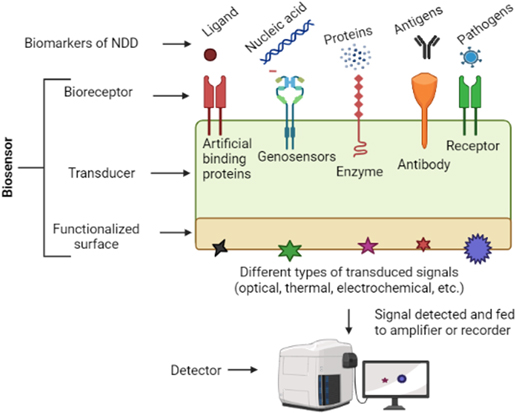
Due to rapid progress in sensor technology, we have access to different types of modern-age sensors for detecting the molecular, cellular, electrical, and behavioural changes associated with the progression of NDD. 17–19 Remarkably, the advent of nanomaterials, electroactive polymers and internet-of-things (IoT) has allowed the creation of miniature sensors with better performance than traditional biosensors for neurological disorders. 17,20–23 In simple terms, a sensor is a technology or apparatus that can detect any input signal and then transform this signal into a quantitative and observable form. The input signal might be any quantity or physical fluctuation that can be measured. In various medical diagnoses, including those involving the real-time detection of human biofluids, biosensors have emerged as cutting-edge detection approaches. 24 The market for biosensors was estimated to be worth USD 25.5 billion globally in 2021. It is anticipated to increase 43%–44% within five years, with the medical and health sector being the most significant segment. 25 A basic idea of the different components of a biosensor is given in Fig. 1. An NDD biosensor is a diagnostic tool that produces an electrical signal when in contact with a target analyte (patient's CSF or blood) or pathogen (responsible for neurological infections) in solution. These are created by fusing a biological detecting element and physicochemical transducer. Biological molecules like antibodies, enzymes, receptors, etc., can act as the recognition or detection elements due to their specific reactivity with the analytes. On the other hand, transducers, which convert a biomolecular interaction into a digital signal, are commonly made up of functionalized electrode surfaces. 26,27
Figure 1. A general idea about the composition of the biosensor. The main surface of the biosensor consists of bio-receptors or recognition elements, which specifically bind to the different biomarkers (like DNA, RNA, proteins, pathogens, etc). The binding reaction changes the functionalized transducer surface at the other end of this biosensor. Depending upon the principle of the biosensing technique, the transduced signal can be optical, mechanical, thermal, or electrochemical, which in turn can be detected using suitable detectors.
Download figure:
Standard image High-resolution imageDepending on its transducer signal, a biosensor can be electrochemical, optical, thermal, or mechanical. A brief description of each type is as follows: In an electrochemical biosensor, the working electrode functions as the transducer. Metals like gold (Au), metal-oxides like titanium dioxide (TiO2) and nonmetals like carbon, which are both conductive and semiconducting, are used to create electrodes. 28 These conducting components are embedded in an insulating polymer or ceramic substrate made of Teflon, glass or polyether ketone. The components of an optical biosensor include a light source, an optical transmission medium, a biorecognition element that has been immobilized (enzyme, antibody, ligand, etc). and a signal-detecting device. These complement one another to measure how a target analyte and ligand interact. The physicochemical conversion (change) produced by the biorecognition process is assessed by observing the amplitude, phase, and frequency of the resultant light.
Thermal biosensors assess the heat energy released during exogenous or absorbed during an endogenous reaction. Since most of the NDD is associated with oxidative stress, the generation of free radicals and the effect of oxidation produces heat. These minute changes in the heat energy can be detected by attaching a thermal biosensor with a suitable amplifier, pH meter, two thermistors and a computer or output device. Further, microelectromechanical systems (MEMS) technology permits the integration and low-cost batch production of small devices with efficient thermal isolation, low thermal mass, and sample volume. Such fabricated devices also make it possible to measure numerous biological samples simultaneously. Mechanical biosensors are based on mechanical interactions generated by cell adhesion, cell motility, molecular affinity, and transport inside a neuron. Biological sensing in the mechanical domain provides exceptional prospects to measure physical characteristics (mass, speed, density, etc.) from cellular to subcellular activities. The moment's need is to integrate nanotechnology for creating biological probes with single-molecule sensitivity and biomolecule size compatibility.
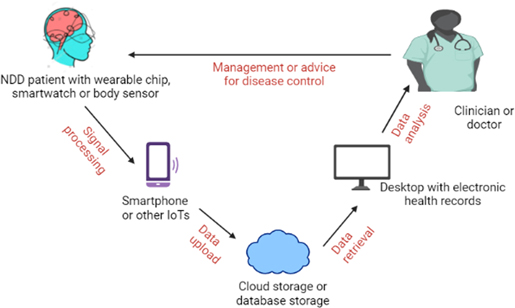
Besides these biosensors, which can be used by medical practitioners or expert researchers to detect changes in biochemical components, we also have implantable and wearable biosensors these days. 29 These implantable and wearable biosensors work almost similar to the principle of general biosensors (Fig. 2). They can be connected to IoT to transmit real-time monitoring data to anyone, like doctors or relatives of the patient. "Wearable GPS and accelerometer" technologies for supervising mobility and physical activity of NDD patients are the most common example of such biosensors. NDD implantable and wearable biosensors have electrodes of conjugated polymers and polymer composites. Polymers offer mechanical properties that enable electrode-tissue mechanical matching, which is crucial for implantable and wearable biosensors. 30
Figure 2. Wearable biosensors in the form of chips, smartwatches, implantable electrodes, etc. need to be attached to the body of NDD patients continuously for real-time monitoring. The nearby smartphones or other IoTs collect the generated signals from these biosensors through a wireless mode. The recorded data is sent to database storage or cloud storage through the internet, which can be accessed by clinicians or doctors for a detailed analysis of the progression of NDD. Finally, doctors or clinicians can prescribe proper management or therapeutic intervention to the patients based on their analysis.
Download figure:
Standard image High-resolution imageMicroelectrodes and signal transduction methods are used in these wearable devices to enable brain-computer interaction. The extraction of brain signals and implantation of signals into neural structures form the foundation of their operation. Researchers created a completely functional brain-computer interface by implanting subdural electrodes and transmitters in the motor cortex. A wearable device for diagnosing neurodegenerative diseases based on gait patterns was created by Saadeh et al. 31 Their results showed that dynamic gait classification had an accuracy of 93.8, 89.1, 94, and 93.3 per cent for amyotrophic lateral sclerosis (ALS), Huntington's disease (HD), Parkinson's disease (PD), and healthy individuals, respectively. Similarly, implantable technology is now accessible for treating NDD through signal insertion into the neural system or brain stimulation. 32
Current Challenges
These advanced biosensors have undoubtedly helped us to detect and manage neurodegenerative disorders to a greater extent. Moreover, they have several advantages over traditional methods of detecting different neurological disorders. They possess long-term stability, high selectivity towards analytes, economical usage, accurate analysis, tiny size, handy design, and wide limit of detection (LOD). 33 Most of these merits of biosensors can be attributed to the integration of state-of-the-art materials in the fabrication of functionalized electrodes or transducer surfaces. 34 In addition, the regular upgradation and modification of electrodes for the synthesis of the transducer has just augmented the merits of the biosensors. There has recently been interest in investigating carbon-based electrodes (such as graphite, carbon nanotubes, and graphene) as potential alternatives to the financially more expensive metallic or ceramic electrodes. 35 It's important to note that because many of these carbon-based materials contain nanoscale structures, they have advantages in terms of nanostructuring.
Despite all these advantages, the usage of biosensors faces hurdles in their practical applications for NDD detection and management. The electrode's physical structure and chemical properties, which are influenced by the material manufacturing process and design, dictate the performance, cost of production, disposal, and sensitivity of biosensors. In addition to the manufacturing process, the verification and standardization of these biosensors are laborious due to different testing standards, various types of sensors, individual differences among the participants or subjects, real applications, ethical issues, and privacy of the patients. 33,36,37 Further, the cost of electrodes can be high due to the use of gold and platinum metals in their making. Sections of larger metal pieces are frequently chopped off to create metal electrodes, which enhance their production costs. 19,38 Similarly, wearables and IoT-connected gadgets have high prices because they need a strong connection and ultralow latency to enable rapid performance in real time.
One also needs to be careful about the possible neurotoxic effects of material or nanomaterial used for synthesizing electrodes. These electrodes may be suitable to use during in vitro conditions. Still, they might be toxic after interacting with the brain cells or CSF due to a combination of defective molecules or free radicals during NDD. 39 Corollary to that, the stability of different materials used in biosensors' electrodes must be checked during different NDD milieu conditions. The stability of electrodes will affect the detection time on one hand, whereas it will produce lots of e-waste on the other side of the coin. Moreover, other issues related to the biocompatibility of any nanomaterial with respect to different brain cell types or fluid would be inadequate due to the complexity of the immune response and healing processes beyond the blood-brain barrier. Therefore, biocompatibility testing, which consists of many in vitro tests, is occasionally laborious. The inability to replace biosensors poses severe difficulties for several biosensors-based NDD monitoring systems. Moreover, the sensitivity of each biosensor is affected by the material used to manufacture, non-specific transduced signals, temperature, and pH of the analyte. 29,39
Understanding the cellular & molecular functioning of the nervous system and figuring out the mechanisms & pathways that lead to neurodegeneration are the main obstacles to the prevention and treatment of NDD. 40 Therefore, using different biosensors for managing or detecting NDD requires consistent background knowledge of human physiology, cognitive dysfunctions, and disease epidemiology. Additionally, the type, size, location and abundance of analyte or biomarker affect the performance of the electrochemical biosensor depending on the specific electrochemical technique used. Since there are fewer blood biomarkers for detecting NDD, their exact detection remains challenging, either through CSF or inside brain regions. 41 The brain is considered the best-protected organ of the body due to the presence of the skull, meninges, blood-brain barrier, and blood-cerebrospinal fluid barrier. These barriers force neuroscientists to go for an invasive method for sample collection and electrode placements. 42 As CSF is the best fluid to analyze the different biological biomarkers associated with the NDD progression, scientists or doctors need to be experts in the collection of CSF samples from the patient's spinal cord, placement of electrodes, the tedious procedure of sample preparation, or controlling the fluctuating measuring conditions.
Prospects
The various shortcomings in using biosensors for NDD can be overcome by integrating nanotechnology in the manufacturing of electrodes. The utilization of nanotechnological tools has modernized NDD biosensing strategies to detect and monitor a diversified range of neuronal changes. 43,44 Particularly, the extensive use of nanoparticles in recent years has led to improvements in sensitivity, detection range, and reaction time. The nervous system can allow nanoparticles of small size, ideal hydrophobicity, charge, and functionalization inside their cells. Thus the improved functionalized biosensors will have a quick reaction time, better monitoring of the biomarkers change and early detection of neurological disorders. 45 Additionally, synthesizing various nanostructures is possible, which can be used to create biosensors with enhanced sensitivity, selectivity, and LOD. These features can offer promising future electronic, electrochemical, and optical sensing systems. 46
Recently, research on using carbon nanoparticles to diagnose Alzheimer's disease has shown that these nanoparticles can interact, detect and damage the amyloid deposits. Similarly, carbon nanotubes or allotropes of graphene are capable of augmenting sensor conductivity. 47 On the other hand, the electrode surface can be functionalized for better transducer signals by electro-spun nanofibers or metallic nanoparticles, which create binding sites for the immobilization of biomarkers or recognition elements. 48 Nowadays, we have molecularly imprinted polymer (MIP), which filters out noise and interacts only with the target analyte to enhance the sensor's selectivity. 49 These examples demonstrate how the adaptability of nanotechnology can be exploited to develop biosensors that function better.
Though the electrochemical sensors are the most economical and trustworthy tools for rapid screening of NDD samples, the increase in the sensitivity of electrodes using nanomaterials will lead to a lesser sample requirement (maybe a single drop of blood, CSF, etc.). Moreover, an attempt to reduce the manufacturing cost of biosensors can be made by utilizing suitable nanomaterials for the transducer surface of the biosensor. 50 The toxicity of the used nanomaterial in a biosensor is a significant concern. Neurotoxicity arises due to many characteristics of nanomaterials, including their chemical composition, surface charge, size, shape, and surface area. By functionalizing nanomaterials with biocompatible materials like polyethylene glycol, using bioinformatics to understand how nanomaterials interact with brain molecules, using green synthesis to create recyclable nanomaterials, and using biocompatible materials to create sensors that can be reused, recycled, & degraded, toxicity can be reduced. 51–53 On the other hand, non-invasive detection of brain functions or dysfunctions during NDD is possible by the advancements in the brain-computer interface, where an encapsulated electrode over the head can record the neuronal activity and pass this information through an amplifier to a computer for detailed analysis. 54
These biosensors' sensitivity, detection range, and response times have significantly increased due to the current widespread use of nanoparticles. 55,56 The different nanotechnological techniques can be used to create a variety of electrodes, from micro- to nanostructures. As a result, electrodes can be divided into groups based on the components utilized, the manufacturing process, and the structure. Designs for electrodes can be array-based, planar, wire-based, nanostructured, and more. 57 Efforts are going on to use the stochastic sensors apart from the enhancement of these classical sensors (potentiometric and amperometric) to explore their possibility in multianalyte assay at very low concentrations. 58
When used as a label or modifier transducer in biosensors, nanomaterials' high load transfer capacity permits lower detection limits and greater sensitivity values. Multiplexed biomarkers detection can become a method for simultaneously identifying phenotypes and various NDD risks. One of the most common methods of achieving multiplexing is employing many transducers, each of which has a unique set of biorecognition capabilities. 59,60 Apart from nanomaterials, polymers can also lead to adjustable electrical conductivity, environmental stability, and biocompatibility. 61 Recently, there have been some promising results from the non-enzyme electrochemical sensor detection technique, which can assist in the early and quick screening of NDD under specific circumstances. It can also be utilized in vivo, allowing for the continuous monitoring of relevant biomarkers in the brain. 27
The analyte recognition elements like antibodies, receptors, enzymes, etc., have also been modified at the nanoscale. The development of nano-receptors based on self-organization and structural flexibility using nanoparticles wrapped in an organic ligand shell is intriguing and promising for biosensors. This ligand-coated shell with a gold core grafting is a flexible array of organic molecules that are organized radially. With this design, the identification of biomarkers is made feasible by interaction sites included in the ligands. 62 On the other hand, Nanobodies are a potent replacement for the bigger size antibodies in the field of medical sciences. Camelids naturally produce antibodies composed only of heavy chains in which the target recognition module is composed of a single variable domain (VHH or Nb). The tiny size, high solubility, high stability, and excellent tissue penetration in vivo of nanobodies are advantageous characteristics. Nanobodies may be chemically coupled to pharmaceuticals, radionuclides, photosensitizers, and nanoparticles at a specific place and are easily genetically connected to Fc-domains, other nanobodies, peptide tags, or toxins. 63 They are ideally suited for the precise and effective targeting of analytes because of these characteristics. Similarly, MXene (Ti3C2Tx)-based enzymatic sensor has the capability for better sensitive, selective and multi-target detection of brain markers. 64
IoT-attached biosensors can be connected to cloud-IoT to offer a free service to the patients by healthcare providers and to make it easier for remote sensors to be operated. The IoT, with a set of sensors, robots, and drones, can be connected to artificial intelligence via the internet. 65 Artificial intelligence-based biosensors can be developed by the integration of flexible nanomaterials and wireless communication, machine learning, & smartphone-based platforms. Access to the 5 G communication system is expected to enhance AI biosensors' productivity by providing better speed and connectivity for wireless communication. 66 This will not only help in the data collection and improvement in the detection accuracy and productivity of treatments; but also help different persons or organizations to monitor the symptoms, analyze the progression of NDD, predict the occurrence of symptoms, and frame government policies for NDD. 67 This will also enable patients to use biosensors to minimize their symptoms, diagnose NDD early, and select the most efficient therapeutic interventions.
Conclusions
Biosensors' applications include disease diagnosis, environmental monitoring, food safety, drug development, biological research, forensics, and many more. However, their use in detecting and managing neurodegenerative disorders is very new. The interaction of biomolecular analytes present in the blood or CSF with a functionalized biosensor electrode produces a detectable and quantifiable signal. Nanoparticles can enhance the biosensors' magnetic, optical, electrochemical, and mechanical signals. Recent studies hint toward biosensors made up of nanomaterials, which can provide a suitable replacement for the conventional, time-consuming techniques of identifying NDD biomarkers. Nevertheless, there are still hurdles in achieving the full potential of advanced biosensors, like the need for advanced material to synthesize electrochemical electrodes, lack of reliable & specific NDD biomarkers, non-specific interactions between bio-analytes and electrodes' material, etc. Therefore, future research must focus on stable and specific electrochemical sensors that can detect NDD and NDD-related biomarkers and find novel composite materials with potent anti-interference properties that can improve the analytical process' sensitivity and specificity.