Abstract
It has previously demonstrated that cholesterol efflux from the cell plasma membrane is increased in a mouse model of cystic fibrosis (CF) compared to a wild-type control. A noninvasive means of characterizing plasma membrane cholesterol efflux at the surface of airway tissue of CF patients is needed to extend the trends found in animal models of CF to the human disease state. Microelectrode-induced cholesterol efflux from the plasma membrane of cells at the surface of tissue is proposed as a strategy to demonstrate increased cholesterol efflux for CF in human subjects. Data demonstrating detection of cholesterol efflux from the human buccal mucosa is reported as proof-of-concept for an in vivo diagnostic assay.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
We are developing methodology to probe cell plasma membrane cholesterol efflux using cholesterol oxidase modified microelectrodes.1–7 This communication reports observation of cholesterol efflux at the human buccal mucosa as proof-of-concept for a diagnostic measurement of cholesterol efflux for management of cystic fibrosis (CF). Previously published work from this research group reported increased cholesterol efflux at the trachea of a CF mouse model.5 A noninvasive human measurement would allow this characteristic to be extended to the human disease state, thus advancing understanding of CF cell biology.
The human measurement is possible because of the small size of the electrode in combination with the double potential pulse data acquisition method. As discussed below, the double potential pulse coulometric scheme allows quantification of only hydrogen peroxide that accumulates at the electrode-tissue interface. An absolute charge for hydrogen peroxide oxidation is measured repeatedly for rapid averaging of data.7 Measuring a rate of microelectrode-induced cholesterol extraction at the tissue surface provides a gauge of the cholesterol concentration in the cell plasma membrane of tissue cells.5
New CF therapies are being developed that target correction of the mutant ion channel, CFTR.8 A specific need in the CF field is the development of a non-invasive marker of disease that correlates with CFTR function and also gives insight into the cellular biology of disease mechanisms. Standard therapy assessment is evaluation of inflammatory markers which is invasive. The determination of membrane cholesterol described here addresses this challenge and represents a significant advancement in biomarker development for the treatment of CF. Cholesterol processing is a biomarker for cellular events that lead to CF clinical manifestations most notably including aggressive inflammation.9 The development of an electrochemical method to detect membrane cholesterol will provide a rapid non-invasive approach to assess how new therapies are targeting pathways key to promoting inflammation and give insight into how secondary manifestations of CF are impacted.
Experimental
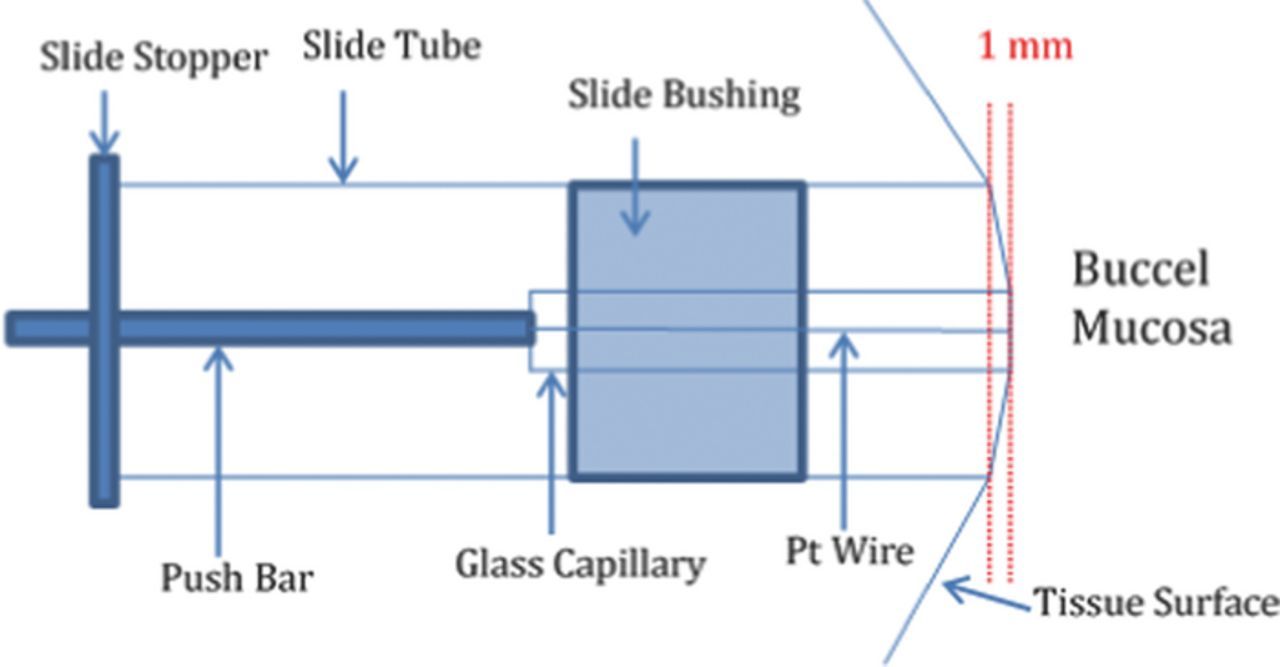
Figure 1 depicts the device and method used to position the electrodes in contact with the human buccal mucosa for IRB approved studies. One end of a slide tube is positioned flush against the inner cheek, isolating and stretching a region of the mucosa with the slide tube held in the patient's teeth. The other end of the tube extends from the oral cavity, through the corner of the mouth so that the microelectrode can be slid through the slide tube and positioned in contact with the tissue surface. The circuit is completed by the human subject inserting the index finger in a beaker containing phosphate buffer and an Ag/AgCl (3 M KCl) reference electrode. The subject is shielded and not grounded.
Figure 1. Hardware design and method for positioning the microelectrode in contact with the human buccel mucosa. Slide tube is held in teeth and stretches the mucosal surface; electrode slides in to contact the tissue.
Carbon disk microelectrodes 10 μm in diameter (CH Instruments) are modified with a submonolayer of platinum nano-crystallites by electrodeposition from a 0.34 mM potassium hexachloroplatinate (Sigma-Aldrich), 0.5 M perchloric acid solution (Sigma-Aldrich). The potential is scanned from 0 V to −0.5 V and back at 2 V/s to deposit a submonolayer of platinum on the carbon electrode. The platinum particles on the carbon electrode surface are modified with cholesterol oxidase by immersing the electrode in 4 mM dimethyl sulfoxide (DMSO) solution of 3,3'-dithiodipropionic acid di(N-hydroxysuccinimide ester) (DTSP; Sigma Chemical Co.) for 45 min. The electrode is rinsed and immersed in cholesterol oxidase solution (1 mg/mL of recombinant cholesterol oxidase (WAKO Chemical USA, Inc., ∼33 units/mg), phosphate buffer pH 6.5 for 3 hours for covalent attachment of the enzyme to the platinum particles on the electrode surface. Additional enzyme is immobilized on the electrode by crosslinking to the initial enzyme covalently anchored to the platinum particles. The electrode is immersed in 8% glutaraldehyde (Sigma Chemical Co.) phosphate buffer, pH 6.5 solution for 30 min and is subsequently immersed in cholesterol oxidase solution for 2 hours.
Chronocoulometric analysis is conducted using a voltmeter-amperometer (Chem-Clamp, Dagan Corp) and a Humbug 60 Hz filter (Dagan Corp). Current integration is performed digitally post-experiment by a program written in-house. An Ag/AgCl (3 M KCl) reference electrode is used for all experiments and all potentials are reported versus this reference potential.
The initial potential (200 mV) is held for 5 s allowing accumulation of hydrogen peroxide at the electrode/tissue interface, as the electrode-immobilized enzyme oxidizes cholesterol. Hydrogen peroxide is not oxidized or reduced at 200 mV, allowing buildup prior to each oxidative detection by double potential pulse. The double potential pulse (both pulses are to 600 mV for 0.250 s) is applied to the electrode for oxidation of the hydrogen peroxide (first pulse) and for gauging the background charge (second pulse). The time between the individual potential pulses of a double potential pulse perturbation is 0.5 s. This potential perturbation waveform is applied for 15 minutes in buffer to condition the electrode. The electrode is immediately placed in contact with the mucosa and the waveform is applied for about one min. (some signal drift is observed) after which three stable difference charge traces are collected and averaged producing a complete measurement. Judicious quality control in sensor production uses bare carbon electrode capacitance, voltammetric evaluation of hydrogen peroxide oxidation activity after platinum deposition, and the double potential pulse difference charge in phosphate buffer. It is noted that variation in total charge for potential pulse application is masked in the double potential pulse difference charge (Supplemental Figure S1).10
Results and Discussion
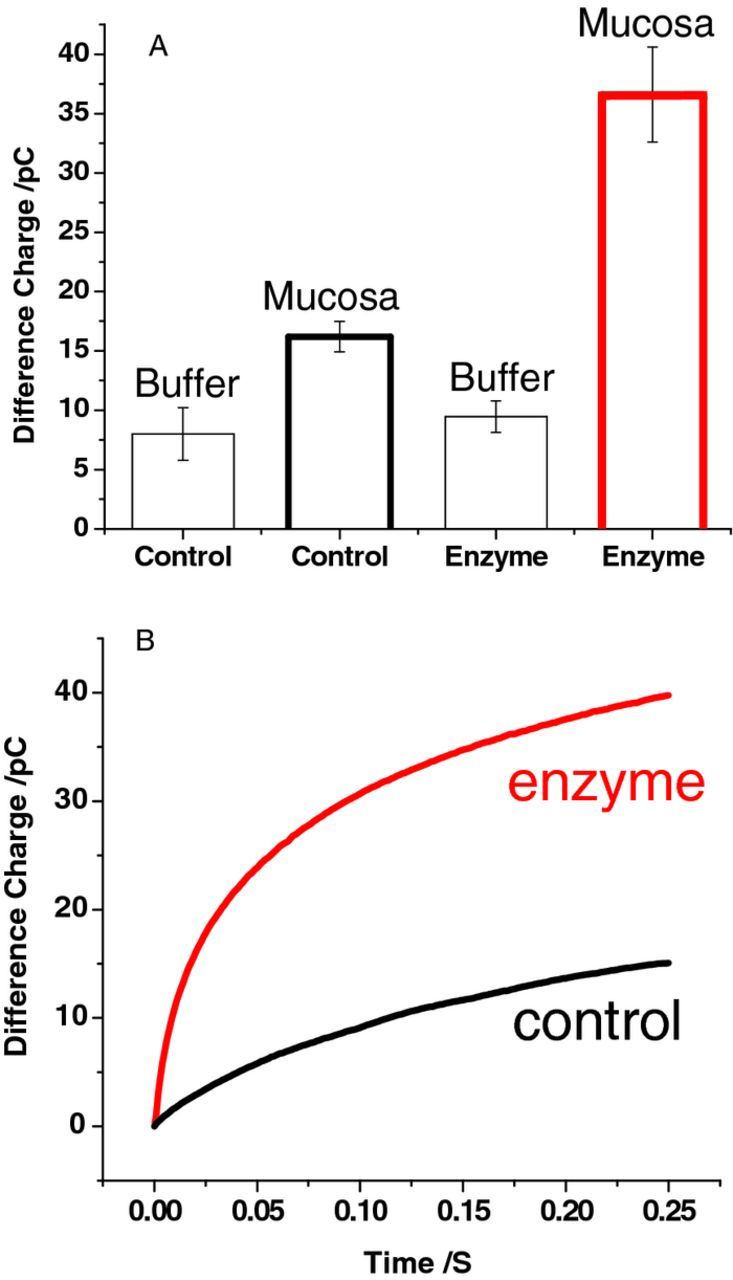
Detection of cholesterol is achieved by averaging sequential double potential pulse coulometric measurements for oxidation of hydrogen peroxide produced by the enzyme. The double potential pulse waveform for detection of cholesterol efflux is discussed in detail in a separate manuscript from this research group7 where it was applied for detection single cell cholesterol efflux. Figure 2a shows a bar graph for data collected at three cholesterol oxidase-modified electrodes and three BSA-modified control electrodes at the mucosa and in buffer. The response for three different enzyme-modified electrodes is 36.7 ± 4.16 pC and the response for three different control electrodes at the mucosa is 16.3 ± 1.12 pC. The enzyme and control electrodes exhibit a statistically different response at the mucosa (average increase for enzyme electrode over control is ca. 20 pC, unpaired student's t-test probability 0.001). The data show a clearly resolvable and repeatable signal reflecting detection of cholesterol efflux from the mucosa membrane. Although the control electrodes show a small increase at the mucosa relative to the buffer response from electro-oxidation/desorption of adsorbed material, the much larger increase observed at the enzyme-modified electrodes at the mucosa, above the buffer response, is assigned predominately to oxidation of hydrogen peroxide produced by the enzyme and thus reflects cholesterol efflux from the mucosa. The enzyme and control electrode responses in phosphate buffer are similar as shown by the bars labeled "buffer". Figure 2b shows an overlay of representative averaged difference charge traces for a cholesterol oxidase modified electrode and a control electrode at the mucosa after initial stabilization.
Figure 2. (A) Bar graph showing the difference charge for three enzyme-modified electrodes and three control electrodes in buffer and at the mucosa. Error bars are SD. (B) Overlay of representative difference charge trace for an enzyme-modified electrode and a control electrode at the mucosa. Traces are the average of three consecutive stabilized difference charge traces for each electrode.
The enzyme modified electrode creates a demand for cholesterol at the site of contact and measures the efflux response. The small site of cholesterol efflux (microelectrode footprint) prevents depletion and allows steady-state extraction of cholesterol through radial diffusion of cholesterol in the plasma membrane of cells and between interstitial fluid between cells at/near the tissue surface. It is estimated that only about 2–3% of the cholesterol present in the cell plasma membrane (directly under the electrode footprint, 78 μm2) is extracted over five seconds for production of each data point. The explanation for being able to detect cholesterol efflux from the plasma membrane at a microelectrode "point of contact" is that the lipid bilayer structure contains about one cholesterol molecule per two phospholipid molecules.11 Furthermore, despite ongoing controversy regarding the rate of transbilayer movement of cholesterol (flip-flop), the outer leaflet of the plasma membrane is believed to be enriched in cholesterol relative to the inner leaflet.12 The concentration of cholesterol in the outer leaflet of the plasma membrane may be as high as 800 mM.13
This experimental achievement is based on our ability to isolate the charge arising from oxidation of enzymatically produced hydrogen peroxide over capacitive and other faradiac charge. The double potential pulse data acquisition scheme is selective to only hydrogen peroxide that accumulates at the electrode contact site and is blind to any hydrogen peroxide that may be present in bulk tissue near the contact site.7 Moreover, the method negates all faradiac steady-state current from diffusional species that react directly at the electrode during the potential pulses. All capacitive charge is also removed minimizing signal drift.7
Cholesterol is generally categorized as being insoluble in water. However, its aqueous solubility has been estimated by our group to be ca. 750 nM by repeated extraction into organic solvent, evaporative concentrating, and LC-MS analysis. With the cholesterol oxidase modified electrode positioned in contact with the plasma membrane surface forming a thin aqueous interfacial layer, equilibrium of membrane cholesterol and aqueous cholesterol is disrupted by enzymatic consumption of cholesterol. A cholesterol concentration gradient across the thin aqueous layer between the tissue surface and the electrode surface is established to sustain a steady-state rate of cholesterol consumption and efflux. Despite the low solubility of cholesterol in the aqueous layer, the short diffusional distance between the mucosa membrane and electrode surface results in an estimated flux of cholesterol to the electrode surface of ca. 20 amol/s.
Summary
Carbon disk microelectrodes containing electrodeposited platinum crystallites covalently modified with cholesterol oxidase allow extraction and detection of cholesterol from the plasma membrane of cells at the surface of the oral human mucosa. The double potential pulse coulometric data acquisition scheme yields a signal of about 20 pC above control electrodes indicating a cholesterol efflux rate from the mucosa of ca. 0.25 amol/s·μm2. The human mucosa cholesterol measurement described in this communication will allow testing of the hypothesis that cholesterol is elevated in inflamed airway tissue of humans suffering from cystic fibrosis.
Acknowledgments
This work was supported by a grant from the National Institute of Health (R01 EB009481) and the Department of Chemistry, Case Western Reserve University.