Abstract
A hydrogen peroxide biosensor was fabricated by immobilization of horseradish peroxidase (HRP) onto the surface of interdigitated microband electrodes. The interdigitated microband electrodes show an amplification factor of ∼9.1 with a collection efficiency of 0.97, demonstrating a high efficiency in generator-collector experiments. Electrochemical impedance spectroscopy was used to follow the biosensor assembly process on the surface of interdigitated microband electrodes step-by-step. When HRP is coated onto the interdigitated microband electrodes, the charge resistance increases significantly, proving the immobilization of HRP on electrode surface. The surface morphology of the HRP modified interdigitated microband electrode was characterized by atomic force microscopy (AFM). AFM images show that the HRP is covering the interdigitated microband electrodes surface evenly. The resulting hydrogen peroxide biosensor exhibits a sensitivity of 114.75 A M−1 cm−2, a detection limit of 9.96 μM, and a linear range up to 500 μM. The performance of the proposed biosensor is evaluated in the presence of potential interferences such as ascorbic acid and uric acid.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
The determination of hydrogen peroxide is important in the fields of food industry, biology, medicine, and environmental protection, and its detection sets the foundation for numerous biosensors and reference methods such as the Trinder reaction.1 Many analytical techniques and commercial test-kits have been developed for such an analysis, including spectrophotometry,2,3 chemiluminescence,4,5 and electrochemical method.6–11 Among these methodologies, electroanalysis provides a convenient way for hydrogen peroxide determination because of its low cost of instrumentation, operation simplicity, high sensitivity, good selectivity, and suitability for real-time detection. Horseradish peroxidase (HRP) is one of the most commonly used enzymes in the construction of hydrogen peroxide biosensors, where it is used at the end of an enzyme chain. This is because the action of highly selective and important oxidases such as glucose oxidase, cholesterol oxidase, and lactate oxidase, leads to the production of hydrogen peroxide, HRP's natural substrate. Monitoring the activity of HRP thus leads to the quantification of the concentration of the oxidase substrate, which is the biosensor primary analytical target. The activity of HRP can be easily measured optically or electrochemically by coupling suitable electron donating chromogens or redox mediators such as ferrocyanide as schematized in reactions 1–3 below.
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/162/7/B133/revision1/jes_162_7_B133eqn1.jpg)
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/162/7/B133/revision1/jes_162_7_B133eqn2.jpg)
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/162/7/B133/revision1/jes_162_7_B133eqn3.jpg)
Moreover, besides its specificity in the reduction of hydrogen peroxide, HRP is highly affordable and readily availability in high purity from a number of sources.12
Microelectrode arrays are powerful tools in electroanalysis because nonlinear diffusion to their edges allows them to offer fast mass transport and quasi-steady state currents. They display many advantageous features such as smaller sample volume, lower interfacial capacitance, lower iR drop, and higher current density than conventional macroelectrodes. Microelectrode arrays can be found in a wide range of geometries, including microbands,13 microrings,14 and microdisks.15,16 Interdigitated electrode arrays are widely used as electrochemical devices because the possibility to polarize each set of microbands separately enables the performance of generator-collector experiments, which result in an electroactive species cycling between two adjacent microbands, enhancing the current response in a stationary solution up to an order of magnitude.17–26 A generator-collector electrode system at interdigitated microband electrodes involves diffusion transport of an electroactive species generated at a generator microband electrode toward an adjacent collector microband electrode which a reverse reaction takes place. Then the original electroactive species will diffuse away from the collector microband electrode toward a generator microband electrode. The electroactive species cycling repeats as long as the electrodes are polarized at potentials above and below, respectively, the formal potential of the redox couple at hand. This cycling makes more electroactive species available near the electrodes resulting in an amplification of the response current. Interdigitated electrode arrays were used to detect viral DNA,27 enzyme activities,28 bacterial cells,29 electroactive species,30 Escherichia coli O157:H7,31 and atrazine.32
The aim of this study is to demonstrate the construction and performance of a hydrogen peroxide biosensor based on the immobilization of HRP onto Au interdigitated microband electrodes. The determination of hydrogen peroxide was performed in the presence of electron mediator ferrocyanide at the HRP modified Au interdigitated microband electrode in generator-collector mode. Hydrogen peroxide in solution is reduced by the HRP(red) to water and HRP(ox) is formed. HRP(ox) can be regenerated with the help of the ferrocyanide which is oxidized in the enzymatic reaction. Finally, the ferricyanide is electrochemically reduced at the electrode surface.33 The results obtained indicated that the immobilization of HRP onto the surface of Au interdigitated microband electrode exhibited good sensitivity for hydrogen peroxide determination. Storage stability and selectivity studies are also presented.
Experimental
Materials and reagents
The reagents used were of analytical grade or the highest commercially available purity and were used as received without further purification. 3-Mercaptopropionic acid (MPA), 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), HRP (261 U mg−1), K3Fe(CN)6, and K4Fe(CN)6·3H2O (≥98%) were purchased from Sigma–Aldrich. Na2HPO4 (≥99%) and NaH2PO4 (≥98%) were purchased from Showa. KCl (99.5%) was obtained from Tedia. N-Hydroxysuccinimide (NHS) was purchased from Alfa Aesar. All samples were prepared with 0.1M phosphate buffer solution at pH 7.0. Deionized water of resistivity not less than 18 MΩ cm was taken from a Milli-Q water purification system (Milli-Q, USA).
Apparatus
A conventional three-electrode system was used, with the Au interdigitated microband electrodes as the working electrode, an Ag/AgCl (3 M KCl) as the reference electrode, and a platinum wire as the counter electrode. Cyclic voltammetry was performed with a CHI 920C Electrochemical Analyzer (CH Instruments, USA). The potential of the generator microband was scanned at 20 mV s−1 while that of the collector microband was held at −0.2 V vs Ag/AgCl. Electrochemical impedance spectroscopy (EIS) measurement was performed with an Autolab PGSTAT30/FRA2 Electrochemical Analyzer (Eco Chemie, Netherlands) at open circuit with a 10 mV amplitude sinusoidal perturbation, applied across the frequency range from 100 kHz to 10 mHz.
Fabrication of the HRP/MPA modified interdigitated microband electrode
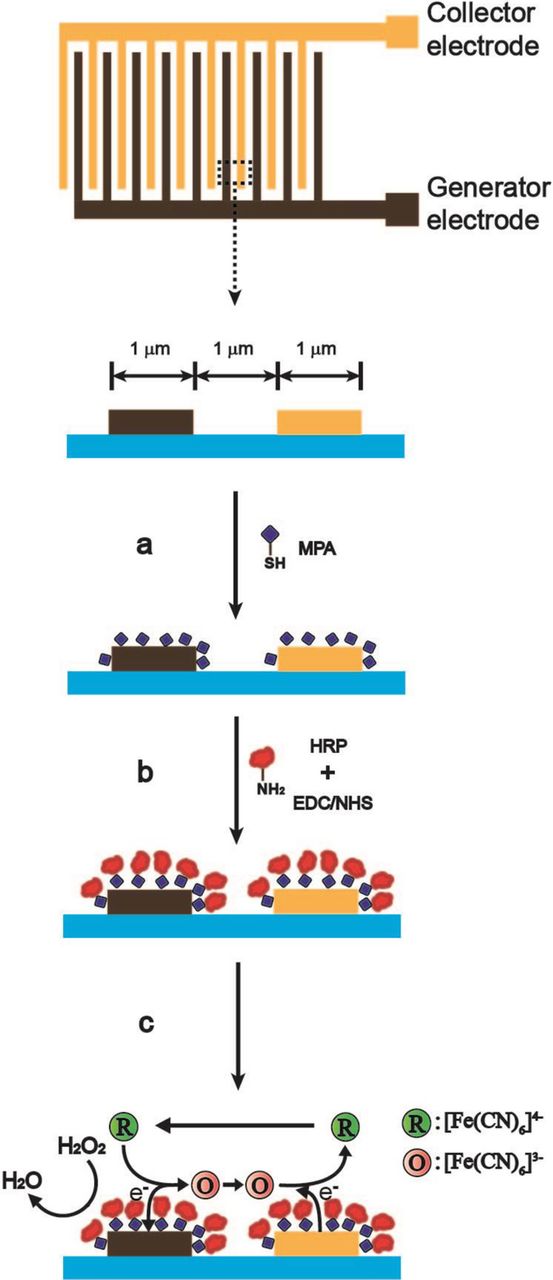
The Au interdigitated microband electrodes with 1 μm band width and 1 μm gap width were fabricated using an optical mix-and-match process, which has been described elsewhere.34 The process combines a step-and-repeat process to define the microband electrodes, and a standard lithography to pattern the active areas and contacts through an SU-8 passivation layer. These are regular interdigitated structures composed by 500 μm long bands on a silicon chip over an active area of 500 μm × 500 μm, that is defined by a passivation layer consisting of a 0.5–2 μm thick SU-8 photoresist layer. These chips were attached and wire bonded to printed circuit boards, and encapsulated using Epotek H-77 thermocurable resin. Before activation, the Au interdigitated microband electrodes were washed serially in ethanol, water, and dried under a nitrogen flow. The electrochemical activation for removing traces of the SU-8 epoxy resin consists of a series of potential steps comprising 5 s at −2 V and another 5 s at 0 V in 0.1 M KCl. After the chips were cleaned, the Au interdigitated microband electrodes were immersed in 0.1 M phosphate buffer solution containing 10 mM MPA for 4 h to form a self-assembly layer. The MPA modified interdigitated microband electrodes were immersed in 0.1 M phosphate buffer solution containing 10 mM EDC and 10 mM NHS as a coupling agent for 1 h. After rinsing with 0.1 M phosphate buffer solution, the modified interdigitated microband electrodes were further treated with 0.1 M phosphate buffer solution containing 2 mg ml−1 HRP for 4 h to form HRP/MPA modified interdigitated microband electrodes. Thus, the sensing interdigitated microband electrodes were obtained. The scheme of fabrication steps and probing mechanism for hydrogen peroxide at the HRP/MPA modified interdigitated microband electrodes using generator-collector mode is shown in Figure 1.
Figure 1. Scheme of the hydrogen peroxide biosensor preparation steps on Au interdigitated microband electrodes and detection mechanism: (a) covalent bonding of MPA (MPA/Au interdigitated microband electrodes), (b) covalent bonding with HRP (HRP/MPA/Au interdigitated microband electrodes), and (c) detection of hydrogen peroxide at HRP/MPA/Au interdigitated microband electrodes using generator-collector mode.
Results and Discussion
Electrochemical characterization of the interdigitated microband electrodes
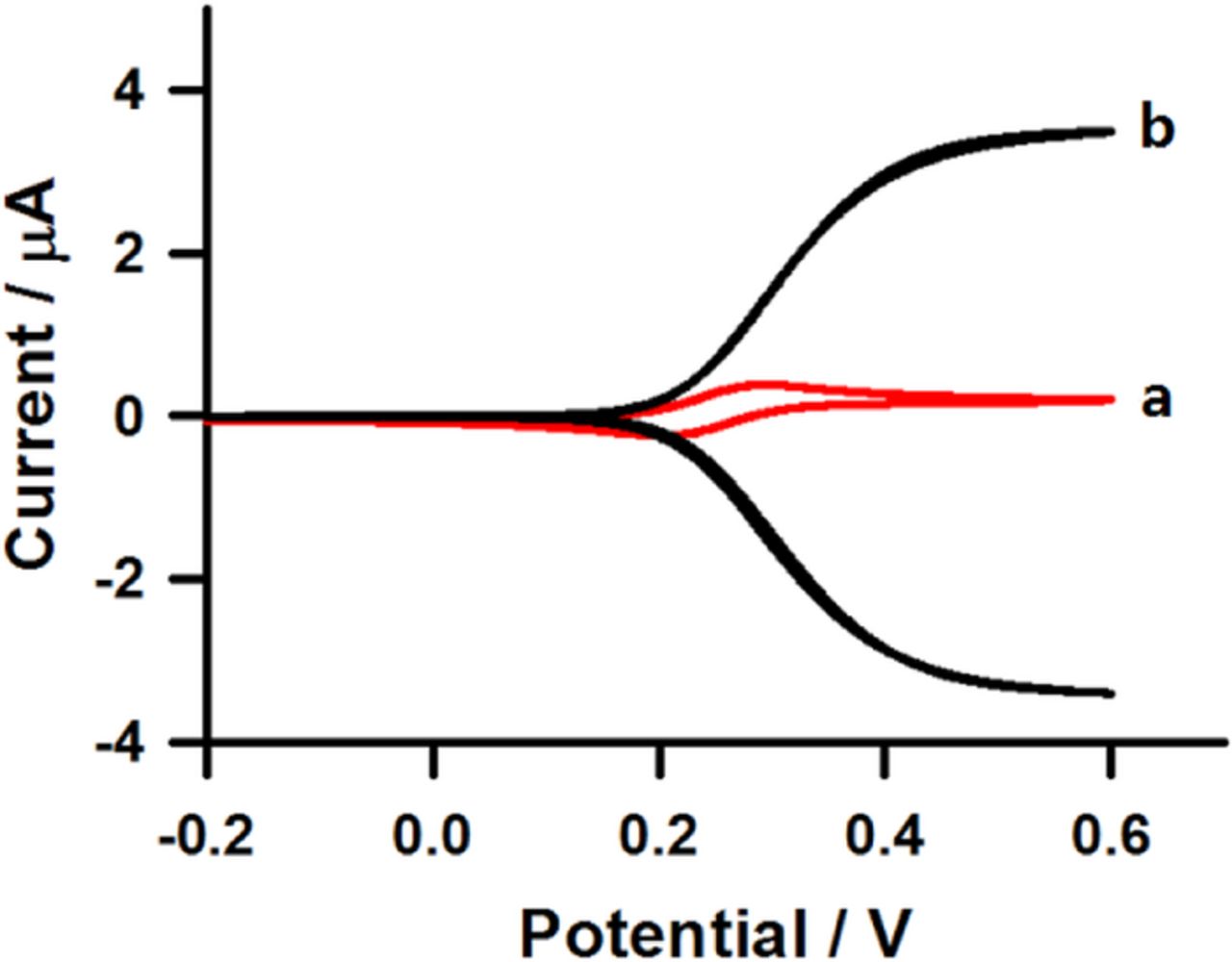
Generator-only and generator-collector experiments were carried out at interdigitated microband electrodes in order to investigate the electrode amplification factor and collection efficiency. The amplification factor is the ratio of the anodic peak current using the generator-only mode to the steady state current recorded at the same electrode operated in generator-collector mode. On the other hand, collection efficiency is the ratio of the steady state current at the collector electrode (icol) which ferricyanide is reduced to ferrocyanide versus the steady state current at the generator electrode (igen) which ferrocyanide is oxidized to ferricyanide using the generator-collector mode. Cyclic voltammograms of generator-only and generator-collector in 0.1 M phosphate buffer solution containing 5 mM ferrocyanide at interdigitated microband electrode are shown in Figure 2. It can be seen that a steady state current is obtained with generator-collector mode (Figure 2, curve b) and that the current at the generator electrode is greatly enhanced compared to that of the generator-only mode (Figure 2, curve a). An amplification factor, which is the ratio of the response current obtained with generator-collector mode to that obtained with the generator-only mode, around ∼9.1 was observed, indicating that the interdigitated microband electrode was highly effective in obtaining a high response current using generator-collector mode. This result is attributed to the rapid diffusion of redox species between the two sets of microbands, which are polarized at potentials above and below the formal potential of the ferrocyanide/ferricyanide couple, respectively; resulting in the continuous transformation of species from the reduced to the oxidized forms and vice-versa. This redox cycling makes more redox species available near the generator-collector electrodes and it offers important current enhancement. The collection efficiency estimated from the icol and igen in Figure 2b was 0.97 which is very close to unity. This is attributed to the small width (1 μm) and gap (1 μm) between the interdigitated microband electrodes. Both the obtained collection efficiency and amplification factor imply the reversibility of the redox species, and a fast electron transfer at the interdigitated microband electrodes using generator-collector mode, and are in excellent agreement with previously reported simulations.34
Figure 2. Cyclic voltammograms of 5 mM ferrocyanide in 0.1 M phosphate buffer solution at interdigitated microband electrodes using (a) generator-only mode and (b) generator-collector mode. The potential of the generator microband was scanned at 20 mV s−1 while that of the collector microband was held at −0.2 V vs Ag/AgCl.
EIS investigation of the immobilization of HRP on the interdigitated microband electrodes
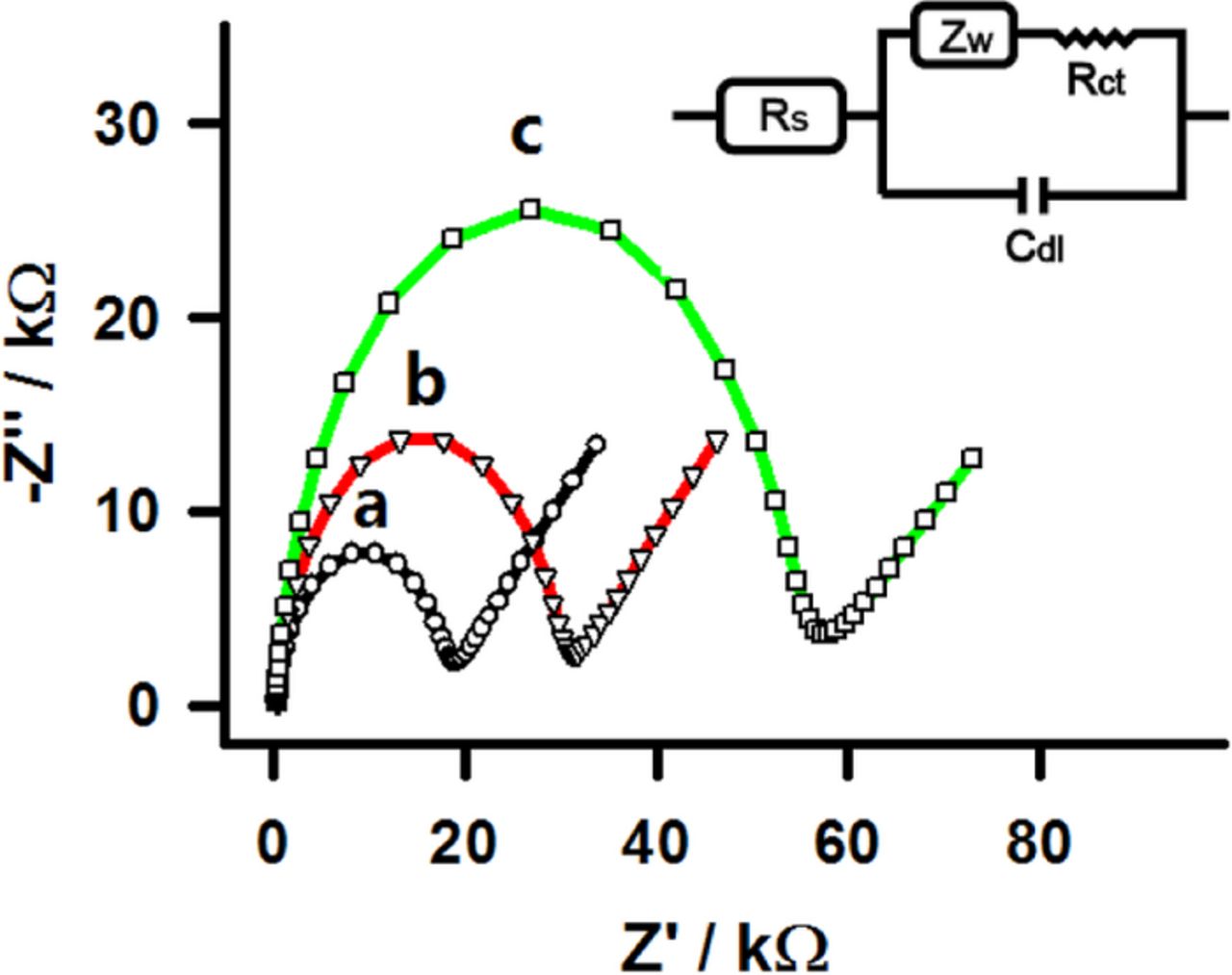
EIS was used to monitor the construction of the biosensor on the interdigitated microband electrodes step-by-step. The Nyquist plots obtained after each preparation step in 0.1 M KCl containing 5 mM [Fe(CN)6]3− and 5 mM [Fe(CN)6]4− are shown in Figure 3. All impedance measurements were performed at open circuit with a 10 mV amplitude sinusoidal perturbation, applied across the frequency range from 100 kHz to 10 mHz. To ensure measurement stability, the EIS experiments for the determination of charge transfer resistance were repeated three times and the relative standard deviation was less than 4%. The Nyquist plot presents a semicircle at high frequencies, consistent with interdigitated microband electrode behavior.29 The Randles equivalent circuit presented in the inset of Figure 3 is appropriate when fitting the experimental data, where Rct represents the charge transfer resistance. The bare interdigitated microband electrodes (curve a in Figure 3) showed a smaller high-frequency semicircle than the modified interdigitated microband electrode, consistent with faster electron transfer (smaller electron transfer resistance). The Rct was about 18 ± 0.7% kΩ for bare interdigitated microband electrodes. When MPA was modified onto the surface of interdigitated microband electrode, the diameter of the semicircle increased clearly and the Rct was about 30 ± 1.3% kΩ (curve b in Figure 3). This is due to the self-assembly of MPA onto the surface of interdigitated microband electrode and effectively excluded the [Fe(CN)6]3−/4−. Then, the MPA modified interdigitated microband electrode was immersed in the HRP solution and this further increased Rct to 57 ± 3.5% kΩ (curve c in Figure 3), suggesting that HRP had been successfully covalently bonded with MPA onto the surface of interdigitated microband electrode and partially blocked its surface. The surface coverage (θ) estimated by θ = (1 - Rct of bare Au interdigitated microband electrodes/Rct of HRP/MPA/Au interdigitated microband electrodes)35 was about 0.68 ± 1.3%.
Figure 3. The EIS of different modified electrodes in 5.0 mM [Fe(CN)6]3−/4− dissolved in 0.1 M KCl recorded on (a) bare Au interdigitated microband electrodes, (b) covalent bonding with MPA (MPA/Au interdigitated microband electrodes), and (c) covalent bonding with HRP (HRP/MPA/Au interdigitated microband electrodes). The measurements were performed at open circuit with a 10 mV amplitude sinusoidal perturbation, applied across the frequency range from 100 kHz to 10 mHz.
AFM characterization of the HRP/MPA modified interdigitated microband electrodes
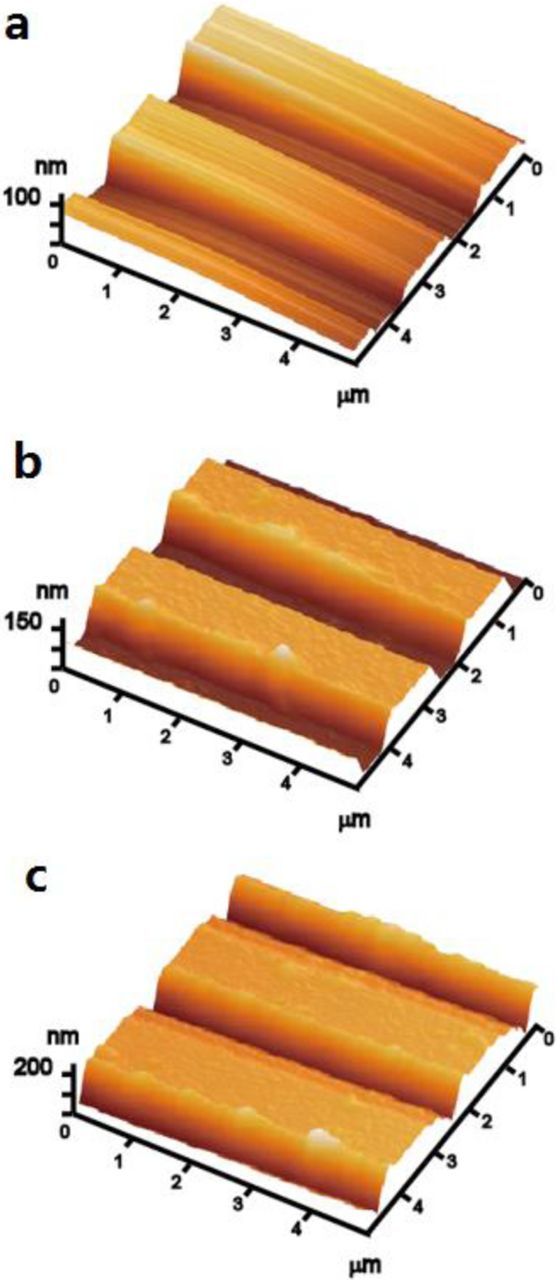
In electrochemical biosensors, at least one biological recognition element is attached to an electrode surface. The surface morphology of the HRP/MPA modified interdigitated microband electrode was characterized by AFM to investigate the assembly process step-by-step on the surface of interdigitated microband electrode. The AFM images of bare interdigitated microband electrode, the same electrode modified with MPA, and finally the HRP/MPA modified interdigitated microband electrode are shown in Figure 4. A typical AFM image of HRP/MPA modified interdigitated microband electrode is shown in Figure 4c. This result presents that the HRP was coated onto the surface of interdigitated microband electrode. From the AFM images we also can conclude that the surface of the HRP/MPA modified interdigitated microband electrode is rougher in Figure 4c than those of bare interdigitated microband electrode in Figure 4a and MPA modified interdigitated microband electrode in Figure 4b. The root-mean-square roughness values are 1.9, 2.5, and 8.2 nm for the bare interdigitated microband electrode, MPA modified interdigitated microband electrode, and HRP/MPA modified interdigitated microband electrode, respectively. This is attributed to the fact that the HRP is immobilized on the surface of interdigitated microband electrode.
Figure 4. AFM images of (a) bare Au interdigitated microband electrodes, (b) covalent bonding with MPA (MPA/Au interdigitated microband electrodes), and (c) covalent bonding with HRP (HRP/MPA/Au interdigitated microband electrodes).
Determination of hydrogen peroxide at the HRP/MPA modified interdigitated microband electrodes
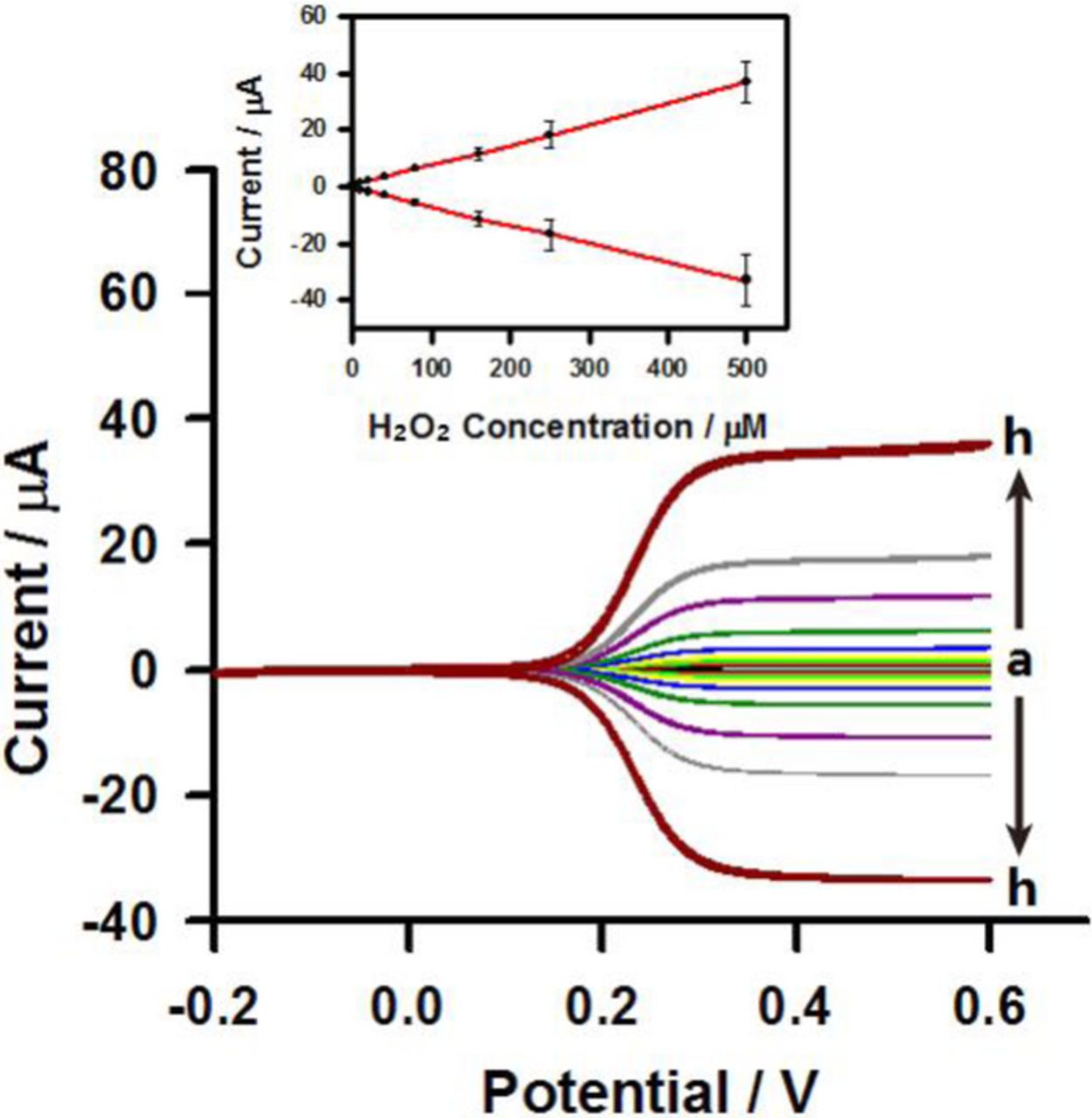
To evaluate the potential application of the prepared HRP/MPA modified interdigitated microband electrodes for the determination of hydrogen peroxide in aqueous solution, different concentrations of hydrogen peroxide were added into 0.1 M phosphate buffer solution (pH 7). The typical cyclic voltammetric responses of the proposed hydrogen peroxide biosensor to successive additions of hydrogen peroxide in 0.1 M phosphate buffer solution (pH 7) containing 5 mM ferrocyanide using generator-collector mode are shown in Figure 5. It can be seen that both the oxidation (generator) and reduction (collector) currents responded sensitively to the addition of hydrogen peroxide and reached steady state at about 0.35 V vs. Ag/AgCl. The corresponding calibration curves for the generator and collector electrodes shown in Figure 5 (see the inset) indicates that the prepared hydrogen peroxide exhibits a linear range up to 500 μM (i (μA) = 0.0717 × c (μM) + 0.4860; r2 = 0.9996), a sensitivity of 114.75 A M−1 cm−2, and a detection limit of 9.96 μM. The detection limit is determined according with the 3 Sb/m criterion, where m is the slope of the linear calibration plot and Sb is estimated as the standard deviation. The sensitivity (114.75 A M−1 cm−2) of the present hydrogen peroxide biosensor is better than previous reported hydrogen peroxide biosensors based on HRP such as adamantane-modified HRP/polythiolated-β-cyclodextrin/Au (109 μA M−1 cm−2)36 and HRP/nano-Au/chitosan-entrapped carbon paste electrode (0.013 A M−1 cm−2).37 The reason for that is that the interdigitated microband electrode performing with generator-collector mode provides access to enhanced rates of mass transport that improve the sensitivity of the biosensor.
Figure 5. Cyclic voltammograms of hydrogen peroxide at concentrations of (a) 5, (b) 10, (c) 20, (d) 40, (e) 80, (f) 160, (g) 250, and (h) 500 μM in 0.1 M phosphate buffer solution containing 5 mM ferrocyanide at HRP/MPA modified interdigitated microband electrodes using generator-collector mode. The potential of the generator microband was scanned at 20 mV s−1 while that of the collector microband was held at −0.2 V vs Ag/AgCl. The error bars represent the SD of three time measurements. The inset shows the corresponding calibration curves.
Selectivity of the hydrogen peroxide biosensor
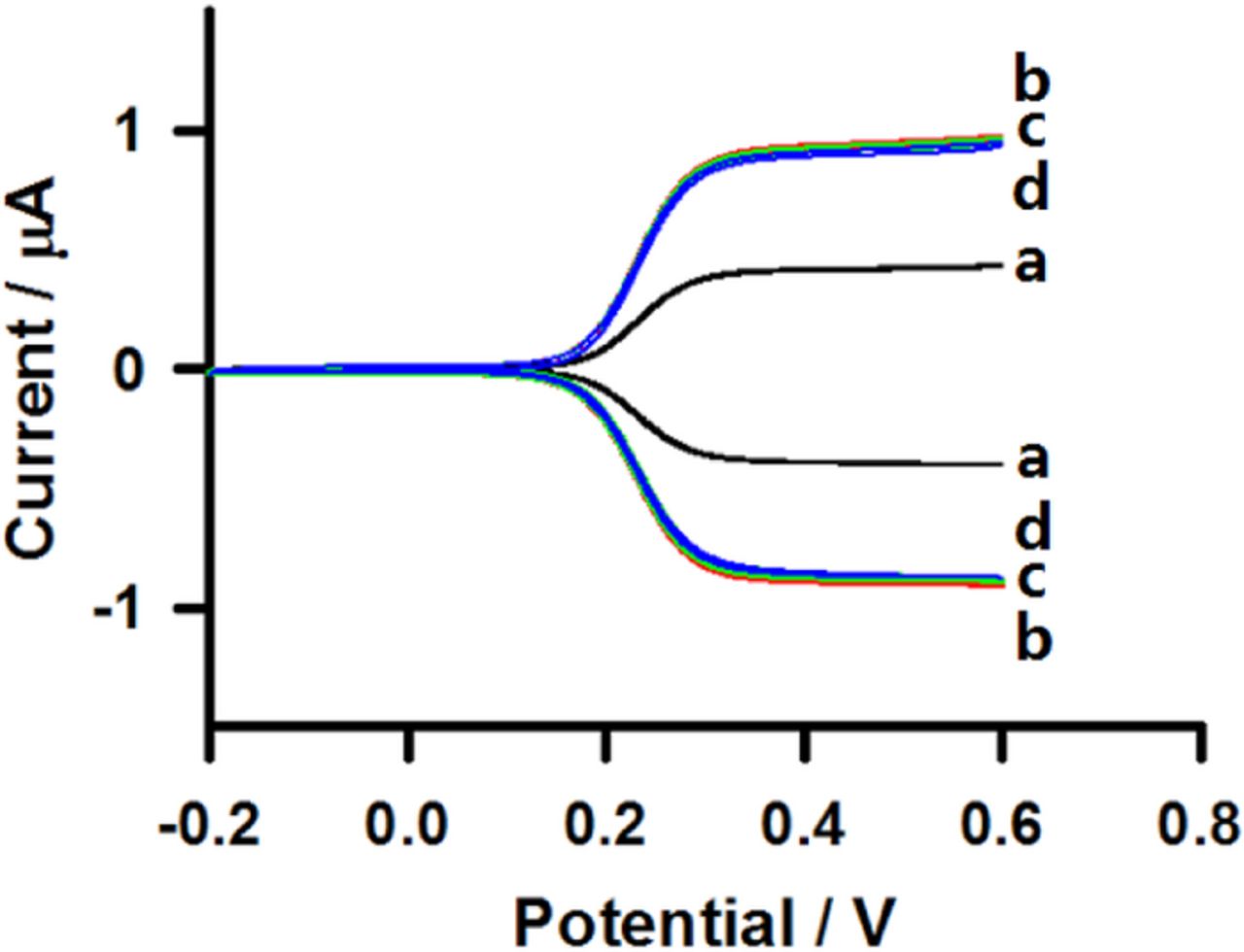
Co-electrooxidation of potential interferences such as ascorbic acid (AA) and uric acid (UA) is a key factor for the evaluation of hydrogen peroxide biosensors. The interference tests were carried out in 0.1 M phosphate buffer solution (pH 7) containing 10 μM hydrogen peroxide in the presence of the same concentration of AA and UA. The responses at the HRP/MPA modified interdigitated microband electrode to the successive additions of 10 μM hydrogen peroxide, 10 μM AA, and 10 μM UA in 0.1 M phosphate buffer solution (pH 7) containing 5 mM ferrocyanide using generator-collector mode are shown in Figure 6. No significant responses can be observed after the additions of 10 μM AA and 10 μM UA to 10 μM hydrogen peroxide in 0.1 M phosphate buffer solution (pH 7) at the HRP/MPA modified interdigitated microband electrode. The results demonstrate that these species coexisting in the sample matrix did not affect the determination of hydrogen peroxide. The reason for this is that the current amplification resulting from operating in generator-collector mode relies on the reversibility of the redox couple involved. On the other hand, UA and AA are irreversibly oxidized, which means that their oxidation products are not reduced back at the collector electrode. This means that their signal is not amplified at all or nearly as much as that of the ferrocyanide/ferricyanide interacting with the HRP, and the result is a selective biosensor with a high selectivity for hydrogen peroxide determination.
Figure 6. Cyclic voltammograms in 0.1 M phosphate buffer solution containing 5 mM ferrocyanide (a) in the absence of hydrogen peroxide and successive additions of (b) 10 μM hydrogen peroxide, (c) 10 μM AA, and (d) 10 μM UA at HRP/MPA modified interdigitated microband electrodes using generator-collector mode. The potential of the generator microband was scanned at 20 mV s−1 while that of the collector microband was held at −0.2 V vs Ag/AgCl.
Storage stability of the hydrogen peroxide biosensor
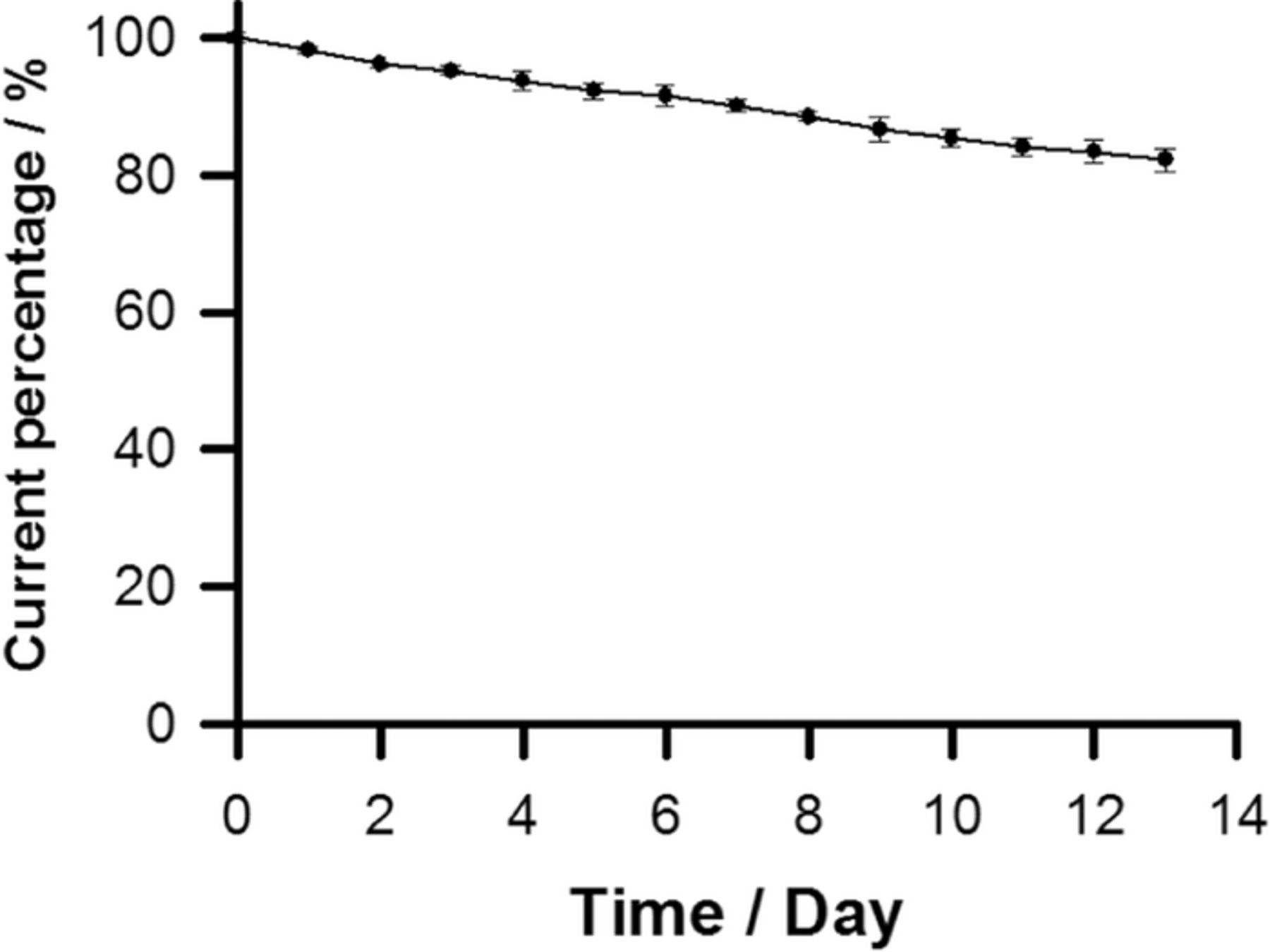
Long-term biosensor stability is crucial for practical application of the proposed hydrogen peroxide biosensor. To evaluate the storage stability of the prepared hydrogen peroxide biosensor, the HRP/MPA modified interdigitated microband electrodes were kept in 0.1 M phosphate buffer solution (pH 7) at 4°C in order to remain its activity when not in use. The stability was examined by periodic measurement of the biosensor responses to 10 μM hydrogen peroxide in 0.1 M phosphate buffer solution (pH 7) containing 5 mM ferrocyanide at the HRP/MPA modified interdigitated microband electrode using generator-collector mode. The change of the generator current at the HRP/MPA modified interdigitated microband electrode for 13 days is shown in Figure 7. It decreased to about 96% of its initial response current on day 3 and about 83% on day 13. The loss of the response current may result from the decrease of the enzyme activity and the materials losses by diffusion into solution during storage.
Figure 7. The change in the response current of 10 μM hydrogen peroxide in 0.1 M phosphate buffer solution containing 5 mM ferrocyanide at HRP/MPA modified interdigitated microband electrodes using generator-collector mode. The potential of the generator microband was scanned at 20 mV s−1 while that of the collector microband was held at −0.2 V vs Ag/AgCl.
Conclusions
A sensitive and selective response to hydrogen peroxide was obtained at the HRP/MPA modified interdigitated microband electrodes working in generator-collector mode. It showed high sensitivity (114.75 A M−1 cm−2) in comparison with the reported values using macroelectrodes and excellent resistance to interferences such as AA and UA. HRP-based biosensors prepared by this method retain their catalytic ability over a period of at least 13 days. The hydrogen peroxide biosensor based on HRP/MPA modified interdigitated microband electrodes can be employed to detect hydrogen peroxide in the analytically important micromolar concentration range. We believe that this is because interdigitated microband electrodes provide access to enhanced rates of mass transport that improve device sensitivity. We have demonstrated that these interdigitated microband electrodes are excellent transducers for the construction of novel and more sensitive miniaturized biosensors. Therefore, the proposed methodology possesses great potential for applications in portable and disposable biosensors. The application of this type of interdigitated microband electrodes to other enzyme systems is in progress.
Acknowledgments
The authors thank the National Science Council, Taiwan under the contract NO. NSC 100-2923-E-005-001-MY3. This work is supported in part by the Ministry of Education, Taiwan under the ATU plan. Also, funding from The Spanish Ministry of Economy and Innovation through project TEC2013-48506-C3-1 is gratefully acknowledged.