Abstract
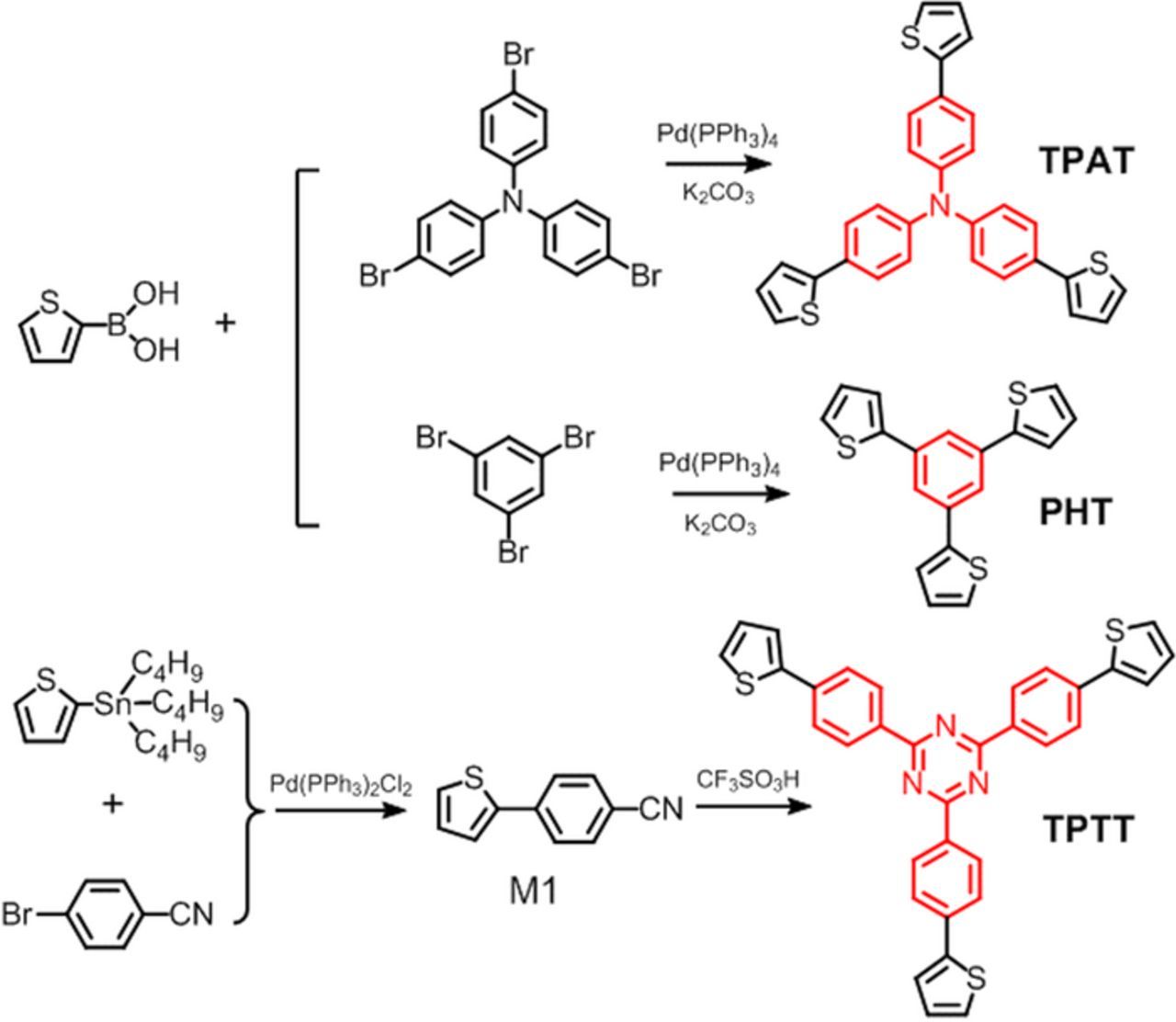
Three star-shaped thiophene derivatives, namely TPAT, PHT and TPTT with different central cores of triphenylamine, phenyl and 2,4,6-triphenyl-1,3,5-triazine respectively, were synthesized and characterized. They were further successfully prepared into the corresponding cross-linked polymers pTPAT, pPHT and pTPTT via electrochemical polymerization. The cyclic voltammetry curves showed that pPHT and pTPTT displayed the relatively higher onset oxidative potentials than pTPAT. After being applied with the positive voltages in an electrochemical cell, pTPAT exhibited the obvious electrochromic behaviors, while such phenomenons were not observed in the case of pPHT and pTPTT. The theory calculation results together with the electrochemistry properties revealed that the electrochromism of pTPAT may mainly depend on its relatively lower oxidative potential because of the introduction of triphenylamine with low ionization energy. Through the copolymerization with 3,4-ethylenedioxythiophene (EDOT) which is also of relatively low ionization energy, pPHT-EDOT and pTPTT-EDOT both exhibited excellent electrochromic properties. The oxidative potential or ionization energy of polymer is thought to be one of the key factors determining the electrochromism.
Export citation and abstract BibTeX RIS
In the past decades, electrochromic (EC) materials have attracted more and more attentions due to their potential applications in smart windows, displays and switchable mirrors.1–3 Among them, organic conjugated polymers (CPs) are viewed as one kind of the most promising materials because of their abundant structure modification, facile processability, fast switching time, outstanding coloration efficiency and color adjustment4–7 and so on. However, for commercial application equipment, novel CPs with superior electrochromic properties are still rare in current day. The essential factor should be attributed to the lack of clearly understanding of the relationship between the molecular structure and electrochromic properties.
Among electrochromic CPs, polythiophenes8–10 have been investigated widely by researchers owing to their rich structural modification induced color richness in neutral or oxidative state as well as the ease of synthesis. The cross-linked polymer network configuration, which is beneficial to the robust stability of the obtained polymer films,11 are attractive to more and more researchers in the design of electrochromic materials.12 Recently, dithienothiophene derivative possessing four thiophene unites at the peripherals was reported to generate cross-linked polymers with excellent electrochromic properties by Arne Thomas' group.13,14 Besides, the cross-linked network may possess some microporous structures in polymer films and thus benefit the electrolyte ions' inserting/deserting behavior during the electrochromic process, which is considered to contribute to the fast switching time.15–17
In this paper, we introduced the triphenylamine, phenyl, and 2,4,6-triphenyl-1,3,5-triazine groups with different electron donating or withdrawing abilities into the central core and three thiophenes for polymerization into their peripheral part to form the star-shaped monomers TPAT, PHT and TPTT respectively (Figure 1). These star-shaped monomers can generate the cross-linked polymer structure via electrochemical polymerization18,19 (Figure 2) and maybe possess some microporous structures in the polymer films, which are expected to help us achieve the excellent electrochromic materials with fast color switching and good film stability. Different central cores with electron donating or withdrawing abilities in the star-shaped monomers may bring us better understanding of the relationship between the structure and electrochromic properties for EC materials.
Figure 1. The molecular structures and synthesis routes of three star-shaped monomers TPAT, PHT and TPTT.
Figure 2. The scheme of electropolymerization reaction and polymeric structure for pTPAT, pPHT and pTPTT with triphenylamine, phenyl and 2,4,6-triphenyl-1,3,5-triazine as central cores respectively.
Three star-shaped thiophene monomers TPAT, PHT and TPTT were synthesized successfully, and then further prepared into the corresponding cross-linked polymers pTPAT, pPHT and pTPTT via electrochemical polymerization. After being applied with the positive voltages in an electrochemical cell, pTPAT exhibited the obvious electrochromic behaviors, while no electrochromism was observed in the cases of pPHT and pTPTT. Theory calculation and electrochemistry results revealed that the electrochromism of pTPAT should been mainly ascribed to the introduction of triphenylamine with low ionization energy. Obtained by electrochemical copolymerization20 with 3,4-ethylenedioxythiophene (EDOT) being of low ionization energy, both pPHT-EDOT and pTPTT-EDOT copolymers exhibited excellent electrochromic properties. These results demonstrate that the oxidative potential or ionization energy of polymer should be one of the key factors determining the electrochromism.21 The experimental data and results will be showed as followed in detail.
Results and Discussion
Synthesis
The terminal compounds TPAT, PHT and TPTT were synthesized according to the synthesis route in Figure 1, and characterized by NMR and Mass spectra. All the reagents or chemicals were commercial products without further purification. 1H (500 MHz) NMR spectra of the synthesized compounds were recorded on Bruker AVANCE III instrument (Bruker, Switzerland). Mass spectra (MALDI-TOF-MS) analysis was recorded using an AXIMA-CFRTM plus instrument.
Tris[4-(thiophen-2-yl)phenyl]amine (TPAT)
Thiophene-2-boronic acid (1.02 g, 8.0 mmol) was mixed with Tris(4-bromophenyl)amine (0.63 g, 1.3 mmol) and K2CO3 (1.21 g, 8.8 mmol) in ethoxyethanol/deionized water (9:1, 15 mL) in a 100 mL two necked round bottom flask. Pd(PPh3)4 (94.0 mg, 0.08 mmol) was added to the stirred suspension, which was then heated rapidly to 130°C and maintained under reflux conditions for 4 hours under a nitrogen atmosphere.22 After cooling to room temperature, deionized water (50 mL) was added to precipitate the main part of the product and then the mixture was wished by water and extracted with dichloromethane consecutively. The product was then dried with MgSO4 and purified on a silica gel column (petroleum ether-CH2Cl2 5:1 as eluent) to obtain the final product as a light yellow powder (0.54 g, yield 84%). MALDI-TOF-MS (M) (m/z):493.1 [M + H]+. 1H NMR (500 MHz, CDCl3) δ 7.53 (d, J = 8.6 Hz, 6H), 7.27-7.25 (m, 6H), 7.17-7.14 (m, 6H), 7.10-7.08 (m, 3H).
1,3,5-Tri(thiophen-2-yl)benzene (PHT)
The synthesis process of PHT was the same as TPAT only used 1,3,5-Tribromobenzene (0.41 g, 1.3 mmol) to replace Tris(4-bromophenyl)amine and PHT was obtained as a white powder (0.38 g, yield 91%). MALDI-TOF-MS (M) (m/z):325.7 [M + H]+. 1H NMR (500 MHz, CDCl3) δ 7.76 (s, 1H), 7.43 (dd, J = 3.6, 1.2 Hz, 1H), 7.36 (dd, J = 5.1, 1.1 Hz, 1H), 7.15 (dd, J = 5.0, 3.6 Hz, 1H).
4-(Thiophen-2-yl)benzonitrile (M1)
4-Bromobenzonitrile (1.46 g, 8.0 mmol) was mixed with (PPh3)4PdCl2 (0.11 g, 0.16 mmol) in 30mL dried tetrahydrofuran(THF) in a 100 mL two necked round bottom flask, then 2-(Tributylstannyl)thiophene (3.58 g, 9.6 mmol) was added, the stirred mixture was then heated to 65°C and maintained under reflux conditions for 16 hours under a nitrogen atmosphere.22 After cooling to room temperature, the mixture was extracted with CH2Cl2 then the organic layer was washed with brine and dried with MgSO4, and purified on a silica gel column (petroleum ether-CH2Cl2 1:1 as eluent) to obtain the product as a white powder (1.39 g, yield 94%). 1H NMR (500 MHz, CDCl3) δ 7.75-7.70 (m, 2H), 7.69-7.65 (m, 2H), 7.44 (dd, J = 3.7, 0.9 Hz, 1H), 7.42 (dd, J = 5.1, 1.0 Hz, 1H), 7.15 (dd, J = 5.0, 3.7 Hz, 1H).
2,4,6-Tri[(5-thiophen-2-yl)-phenyl]-1,3,5-triazine (TPTT)
After added 40 mL dried CHCl3 to dissolve M1 (1.11 g, 6 mmol) in a 250 mL eggplant-shaped flask, CF3SO3H (3.60 g, 24 mmol) was added dropwise at 0°C and stirred for 0.5 h, after that removed the flask to room temperature and stirred overnight. Added 70 mL deionized water to the flask and stirred for half an hour, then NaOH solution was dropwise added to neutralize the mixture until pH = 7. Then extracted the mixture with CH2Cl2 and brine, dried the organic layer with MgSO4 and purified on a silica gel column (petroleum ether-CH2Cl2 5:3 as eluent) to obtain the final product as a white powder (0.17 g, 15%). MALDI-TOF-MS (M) (m/z):555.7 [M + H]+. 1H NMR (500 MHz, CDCl3) δ 8.81 (d, J = 8.4 Hz, 2H), 7.91-7.81 (m, 2H), 7.52 (dd, J = 3.6, 1.0 Hz, 1H), 7.41 (dd, J = 5.0, 0.8 Hz, 1H), 7.18 (dd, J = 5.0, 3.6 Hz, 1H).
Electrochemical polymerization
The polymers of pTPAT, pPHT and pTPTT were fabricated to show as films by electrochemical polymerization from the monomers TPAT, PHT and TPTT respectively. Due to the three thiophenes in the peripheral part of monomers, the obtained polymers would obviously show as the cross-linked network structure as shown in Figure 2. And owing to the relatively rigid structural configuration, these cross-linked network polymers may possess the possible microporous structure in them. The cross-linked network and possible microporous structure can make these polymers exhibit good electrochromic properties.
The electrochemical polymerization was carried out on ITO-coated glasses (9 mm × 20 mm) by the cyclic voltammetry polymerization method via applying a continuous scanning voltage at a potential scan rate of 400 mV/s in a conventional three-electrode cell with 0.1 M Tetrabutyl-ammonium hexafluorophosphate (TPAPF6) in Acetonitrile(ACN)/CH2Cl2 (1:1, by volume) as the electrolyte solution. Electrochemical polymerization of the monomers, and electrochemical properties of the corresponding films were performed on CHI660E electrochemical analyzer (Chenhua, China). Different from each other for TPAT, PHT and TPTT during the cyclic voltammetry polymerization process were the applied voltage ranges of −0.3 V∼1.5 V, −0.3 V∼1.6 V and −0.3 V∼1.5 V on them respectively, which were tightly related to the corresponding potential of their first coupled oxidative-reduction peaks. Figure 3 showed the cyclic voltammetry curves during the electrochemical polymerization process of TPAT, PHT and TPTT. The current intensity in their cyclic voltammetry curves rose up gradually as the scanned cycle number increased, which clearly demonstrated that these monomers underwent the effective polymerization reaction with their corresponding polymers formed and successfully deposited on ITO.23
Figure 3. The cyclic voltammetry curves of the electrochemical polymerization process for TPAT, PHT and TPTT.
Electronic energy
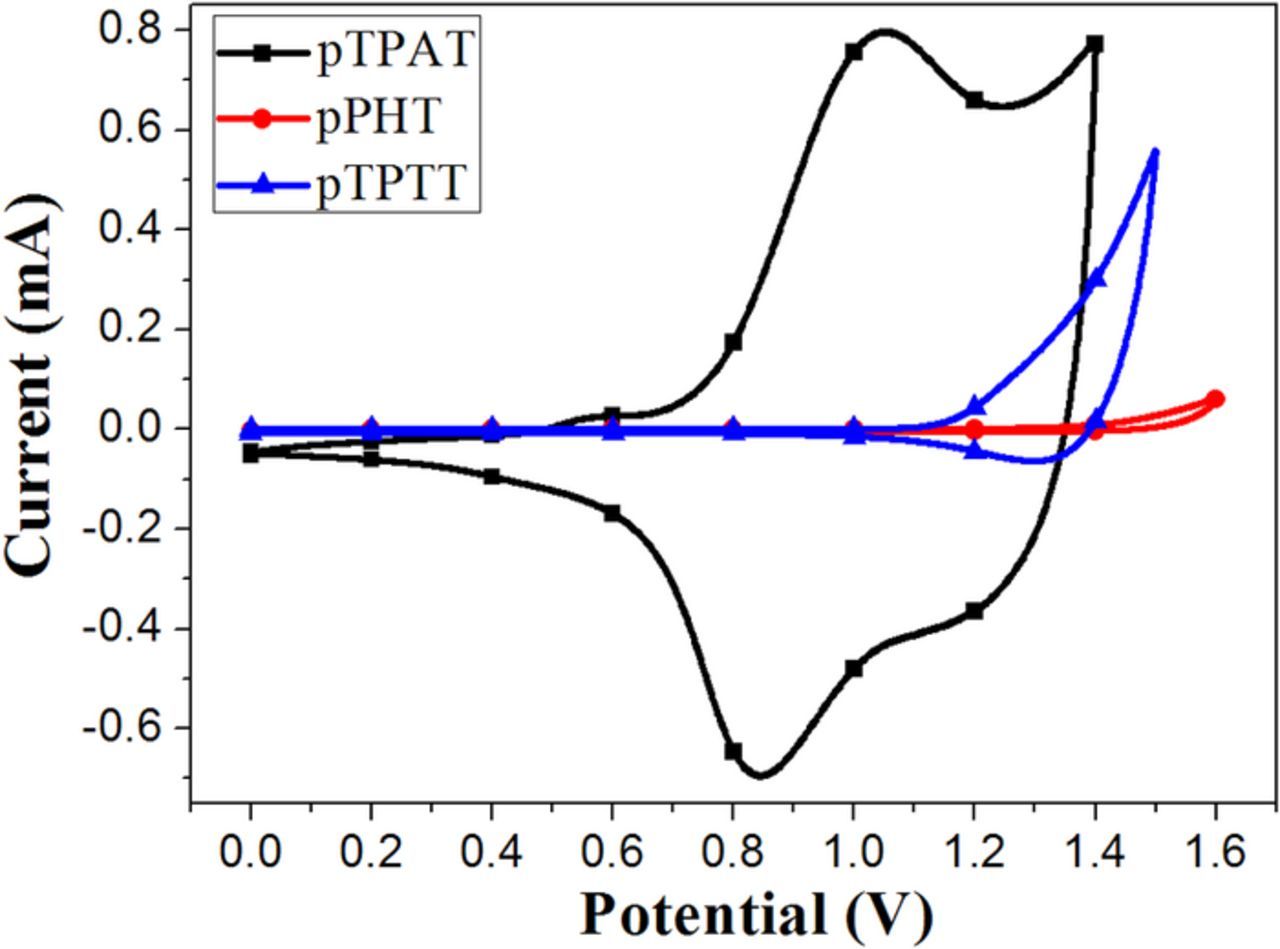
The electronic energy levels of the oxidative states for the obtained polymers pTPAT, pPHT and pTPTT were measured by the cyclic voltammetry electrochemistry method in film state as shown in Figure 4. The onset oxidative potential of three polymers pTPAT, pPHT and pTPTT were obtained to be 0.76 V, 1.18 V and 1.28 V respectively. Obviously, the onset oxidative potential of pTPAT was much lower than those of pPHT and pTPTT. Moreover, the current intensity of pTPAT was much higher than those of pPHT and pTPTT at the first-couple oxidative-reduction peak, which indicated the more injected charge in the pTPAT polymer. All these results demonstrated the easier being oxidized under relatively lower applied voltage for pTPAT polymer film. The key reason may be attributed to the introduction of triphenylamine with low ionization energy into the central core.24
Figure 4. The film cyclic voltammetry curves of the obtained polymers pTPAT, pPHT and pTPTT.
In order to further investigate the electronic character, the electronic cloud distribution and energy level of the electronic transition orbital for three monomers TPAP, PHT and TPTT were calculated by B3LYP method25,26 as shown in Figure 5. The highest occupied molecular orbital (HOMO) of TPAT with an energy level of −4.8 eV was mainly distributed at the triphenylamine part, while those of PHT and TPTT were mainly delocalized at the phenyl thiophene part,27 resulting in their much lower energy level lying at −5.7∼5.8 eV. Thus the introduction of donor triphenylamine with strong electron donating ability or low ionization energy into the central core of star-shaped molecule may effectively enhance the molecular electronic cloud density and thus decrease the molecular oxidative potential. Meanwhile, from the lowest unoccupied molecular orbital (LUMO) of TPAP, PHT and TPTT, another conclusion can be summarized that the introduction of strong acceptor 2,4,6-triphenyl-1,3,5-triazine into the central core of star-shaped molecule may effectively decrease the molecular LUMO level or reductive potential with relatively much weaker effect on the HOMO level or oxidative potential. The theoretical calculation results further demonstrated that the key reason for pTPAT being easier oxidized under relatively lower applied voltage should be mainly attributed to the introduction of triphenylamine, which were consistent with the measured electrochemistry energy results.
Figure 5. The calculated molecular energy levels and corresponding electronic orbitals pictures of HOMOs and LUMOs for monomers TPAT, PHT and TPTT.
Electrochromic properties
The electrochromic properties of pTPAT, pPHT and pTPTT polymer films were measured by carrying out the spectroelectrochemical experiment in ACN solution with 0.1 M TPAPF6 as electrolyte. Spectroelectrochemistry test were investigated by Shimadzu UV-1800 spectrophotometer (Shimadzu, Japan) integrated with the CHI660E electrochemical analyzer. As shown in Figure 6, pTPAT exhibited a maximum absorption peak at ∼425 nm at the neutral state (under 0 V), and the polymer film appeared to be light yellow color. With the increase of the applied voltage, the peak intensity at ∼425 nm decreased obviously and two new absorption peaks rose up gradually at ∼650 nm and ∼1100 nm, which may be attributed to the electron transition of 'polaron' and 'bipolaron' respectively.28 The 'polaron' and 'bipolaron' derived from the electron extractions or hole injections of polymer pTPAT. In this progress, the color of pTPAT film turned to gray eventually at the voltage of 1.5 V as shown in the inset photos of Figure 6a. The UV spectra of pPHT and pTPTT polymers exhibited the nearly same unfeatured absorption character in the range of 380∼500 nm at the neutral state (0 V) with the films showing as the weak yellowish color. In contrast to pTPAT, the obvious absorption peak at ∼425 nm was not observed in the UV spectra of pPHT and pTPTT polymers. It indicated that this special absorption peak at ∼425 nm in UV spectra of pTPAT should be attributed to the introduction of donor triphenylamine which resulted in the enhance of molecular conjugation in pTPAT polymer. Upon increase of the applied voltage, it is surprising that electrochromism effects were not obviously observed with nearly none changes of the UV spectra and exterior colors for both pPHT and pTPTT polymer films. Actually, the current-voltage curves in Figure 3 clearly demonstrated that they underwent the obvious reverse oxidative and reductive reaction during the cyclic voltammetry polymerization process,29 which meant that the electrochromism phenomenon of their polymer films should also appear during the electrochemical polymerization process. In fact, clear electrochromism between light yellow and gray color appeared during the electrochemical polymerization process of pTPAT, while nearly none obvious electrochromism phenomenon was observed for the polymer films of pPHT and pTPTT during their electrochemical polymerization process. In order to further verify the electrochromism of pPHT and pTPTT, the same experimental conditions as their electrochemical polymerization process were applied to measure their UV spectra under different voltages with 0.1M TPAPF6 in ACN/CH2Cl2 (1:1, by volume) mixture solution as electrolyte. As shown in the inset curves of Figures 6b and 6c, pPHT almost exhibited the same UV spectra to the previous one, while pTPTT showed little difference with UV spectra intensity around ∼630 nm and ∼1000 nm enhanced as the voltage increased, though the change of exterior color of pTPTT film was still not very obvious. The electrochromism color and discolor switching time of pTPTT film at 630 nm were roughly estimated to be 1.2 s and 4.7 s respectively30 (Figure 7a), and the electrochromism optical contrasts of pTPTT film were obtained to be only around 10% at both 630 nm and 995 nm, which also decreased gradually as time delayed (Figure 7b). These results reflected that the observed electrochromism effect of pTPTT film in the UV spectra was unstable. From the UV spectra character, the peaks at 630nm should be attributed to the absorption spectra of pTPTT 'polaron' (or oxidative state), which remained in the spectra of pTPTT under 0V as shown in the inset curves of Figure 6c. It indicated that pTPTT film in ACN/CH2Cl2 (1:1, by volume) mixture solution with 0.1M TPAPF6 as electrolyte possessed the relatively high level doping of oxidative state (accompanied by the inserted electrolyte anions) in its film under 0 V. This might benefit the injection of holes into the pTPTT film and contributed to the observed unstable electrochromism in UV spectra. Therefore, the nearly none or weak electrochromism effect for pPHT and pTPTT polymers may be ascribed to their relatively high oxidative potentials as well as the possible insufficient injected holes in films, which could also be seen from their relatively lower current levels in their common cyclic voltammetry electrochemistry curves (Figure 4). These results also demonstrated that the electrochromism effects of pTPAT polymer should mainly derive from the oxidation behavior of triphenylamine cores under the applied voltages. This is consistent with the previous conclusion that pTPAT being easier oxidized under relatively lower applied voltage in comparison with pPHT and pTPTT polymers should be mainly attributed to the introduction of triphenylamine.
Figure 6. The UV absorption spectra and film photos under different voltages between 0 V and 1.6 V for the polymers a) pTPAT, b) pPHT and c) pTPTT in the electrochemical cell with 0.1 M TPAPF6 in ACN solution as electrolyte; inset curves of b) and c) are the UV spectra under different voltages for pPHT and pTPTT in the electrochemical cell with 0.1 M TPAPF6 in ACN/CH2Cl2 (1:1, by volume) solution as electrolyte respectively.
Figure 7. a) Electrochromic switching response of pTPTT films at 630 nm and b) the optical contrasts of pTPTT polymer film at 630 nm and 995 nm (in ACN/CH2Cl2 (1:1, by volume) solution containing 0.1 M TPAPF6 between 0 V to 1.5 V with a residence time of 10 s).
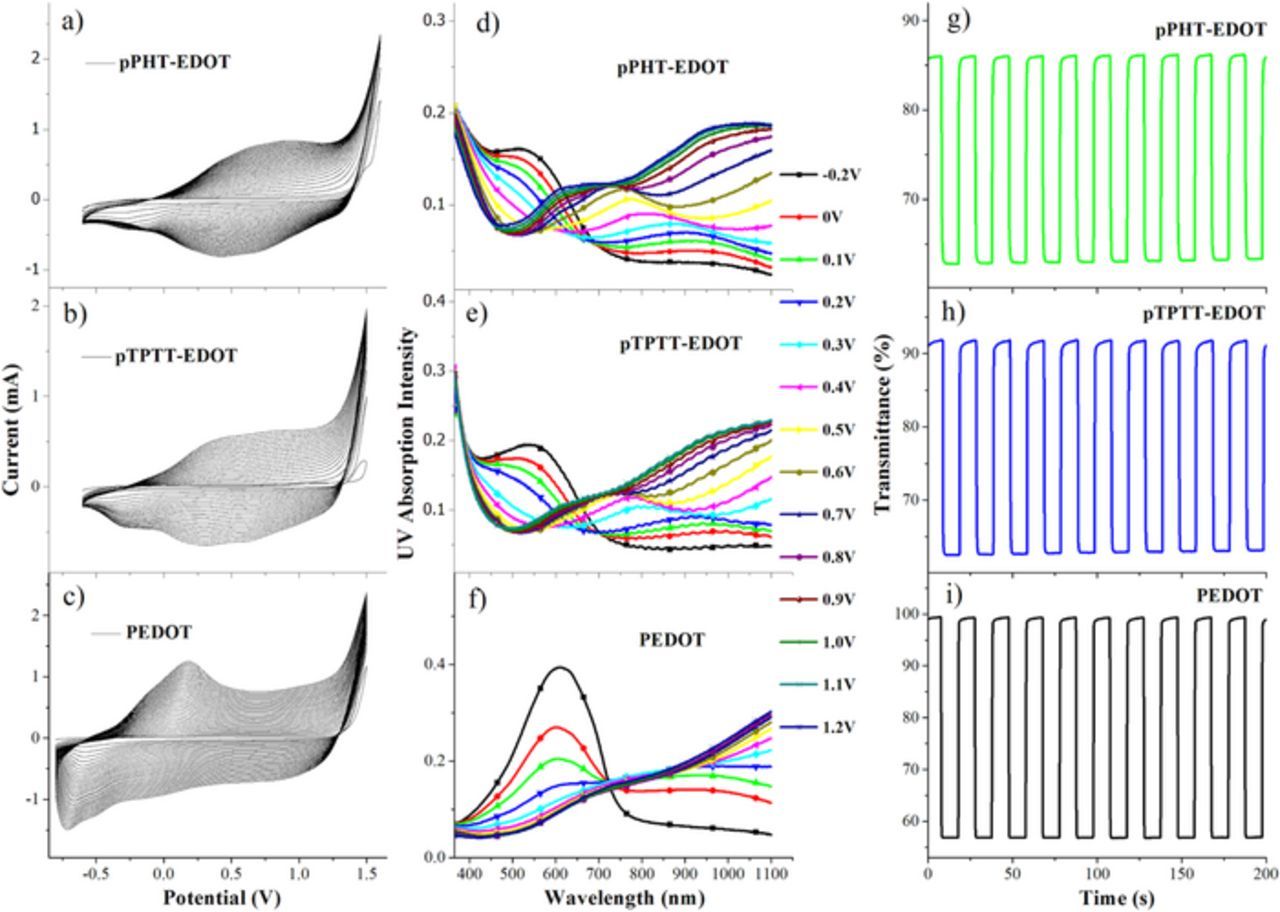
By introducing 3,4-ethylenedioxythiophene (EDOT) which is also of relatively low ionization energy31,32 into the monomers to form the mixture (ratio of 1:1) solutions of PHT-EDOT and TPTT-EDOT, copolymers of pPHT-EDOT and pTPTT-EDOT were fabricated by electrochemical polymerization method under the same experimental conditions to their corresponding homopolymers of pPHT and pTPTT. And since EDOT was used as the comonomer during the copolymerization, we also measured the electrochromic properties of its polymer PEDOT under the same experimental conditions. As shown in Figures 8a, 8b and 8c, the obtained copolymers of pPHT-EDOT and pTPTT-EDOT both exhibited obviously different clectrochemical redox behaviors to their corresponding homopolymers and PEDOT. The oxidative potentials of the copolymers were lower than their corresponding homopolymers and higher than PEDOT. After being applied with the voltages from −0.2 V to 1.2 V, two copolymers pPHT-EDOT and pTPTT-EDOT both showed the obvious electrochromic effects, with the original peaks around 550 nm decreasing and two new peaks appearing at 700 nm and 1100 nm in their UV spectra as shown in Figures 8d and 8e. And as shown in Figure 8f, the homopolymer PEDOT showed the original peak around 620 nm decreasing and one new peak appearing at 1100 nm. The differences of the cyclic voltammetry curves and UV spetra between the copolymers and the homopolymers proved the successful copolymerization of the copolymers pPHT-EDOT and pTPTT-EDOT. Meanwhile, the copolymers' electrochromism optical contrasts were measured to be 23% with the color (discolor) switching time of 0.7 s (0.8 s) at 1100 nm under 1.5 V for pPHT-EDOT and 29% with the color (discolor) switching time of 0.8 s (0.5 s) at 1100 nm under 1.5 V for pTPTT-EDOT as shown in Figures 8g and 8h. The electrochromism optical contrasts of PEDOT were measured to be 43% with the color (discolor) switching time of 0.3 s (0.7 s) at 1100 nm under 1.2 V as shown in Figure 8i. The better performance of electrochromism for copolymers pPHT-EDOT and pTPTT-EDOT than their corresponding homopolymers pPHT and pTPTT should mainly be attributed to the introduction of EDOT unit, further indicating the importance of polymers' oxidative potentials to their electrochromic properties.
Figure 8. The cyclic voltammetry curves of the electrochemical copolymerization process for a) PHT-EDOT, b) TPTT-EDOT and the electrochemical homopolymerizaition process for c) EDOT in the electrochemical cell with 0.1 M TPAPF6 in ACN/CH2Cl2 (1:1, by volume) solution as electrolyte; the electrochromic UV absorption spectra under different voltages between −0.2 V and 1.2 V for copolymers d) pPHT-EDOT, e) pTPTT-EDOT and for homopolymer f) PEDOT, and the optical contrasts at 1100 nm between 0 V to 1.5 V with a residence time of 10 s for copolymers g) pPHT-EDOT and h) pTPTT-EDOT and between −0.8 V to 1.2 V with a residence time of 10 s for homopolymer i) PEDOT (in ACN solution containing 0.1 M TPAPF6).
Conclusions
Three star-shaped thiophene derivatives, namely TPAT, PHT and TPTT with different central cores of triphenylamine, phenyl and 2,4,6-triphenyl-1,3,5-triazine respectively, were synthesized and characteriaed. They were further successfully prepared into the corresponding cross-linked polymers pTPAT, pPHT and pTPTT via electrochemical polymerization. The polymers pPHT and pTPTT displayed the relatively higher onset oxidative potentials than pTPAT. And pTPAT exhibited the obvious electrochromic behaviors, while no such phenomenons were observed in the case of pPHT and pTPTT. Theory calculation results together with the electrochemistry properties revealed that the electrochromism of pTPAT may mainly depend on its relatively lower oxidative potential because of the introduction of triphenylamine with low ionization energy. Through the copolymerization with EDOT being of relatively low ionization energy, copolymers pPHT-EDOT and pTPTT-EDOT both exhibited excellent electrochromic properties. The oxidative potential or ionization energy of polymer is thought to be one of the key factors determining the electrochromism.
Acknowledgments
The authors gratefully appreciate for the support from the National Natural Science Foundation of China (51603185, 51673174, 51573165, 51273179), Zhejiang Provincial Natural Science Foundation of China (LY17E030001, LY15E030006), and the special fund for scientific research from Zhejiang University of Technology (3817101101T).