Abstract
The present study was designed to determine the effect of annealing temperature on the sol-gel synthesized YTixOy electroceramic sensing membrane on silicon substrate as an electrolyte-insulator-semiconductor (EIS) based pH sensor. We observed that the YTixOy sensing membranes after annealing at high temperatures (700, 800 and 900°C) modify the structural feature, sensing characteristic and reliability. X-ray diffraction, atomic force microscopy and X-ray photoelectron spectroscopy have been used to reveal the film microstructure, surface morphology and chemical composition, respectively. As compared to the other annealed samples, the YTixOy sensing membrane annealed at 800°C manifested the highest sensitivity (58.52 mV per unit of pH), lowest hysteresis voltage (2.6 mV), and lowest drift rate (0.10 mV/h). These results demonstrate that the film annealed at 800°C suppresses the formation of oxygen vacancy and intrinsic defect forming a stoichiometric YTixOy sensing film.
Export citation and abstract BibTeX RIS
Several kinds of biochemical and metabolic reactions in the human body rely strongly on the pH of an extracellular environment. A minute variation in the pH of blood plasma has a major impact on the structure of biomolecules and catalytic activity of various enzymes, which lead to severe ailments like acidosis and alkalosis in diabetes mellitus, hence the accurate determination of pH is quite essential in medical diagnostic industries.1 Since the innovation of ion-sensitive field-effect-transistor (ISFET) in 1970 was proposed by Bergveld, most attention has been focused on the fabrication of silicon-based biosensor for detection of different biomolecules, including pH, glucose, urea, creatinine, DNA, RNA.2–5 The concept of ISFET was implemented using metal-oxide field-effect-transistor (MOSFET). However, it is achieved that the gate of MOSFET associated with the ion-sensitive layer accompanied by reference electrode introduces in the electrolyte solution. An ISFET device exhibited several main advantages, such as quick response, smaller size, and label-free detection. The high sensitivity of this device majorly depends on the transducer surface at which analytical biomolecule binds to specific recognition molecule, resulting in allowing local charge transfer due to the current change caused by the phenomena of field effect.6 Recently, electrolyte-insulator-semiconductor (EIS) based sensor has been frequently exploited over ISFET due to its simple, inexpensive fabrication process (without source and drain electrode), label-free, and miniaturized point of care technology. The sensing surface of an EIS biosensor plays a very vital role in the development of reliable and stable sensor; hence, the selection of sensing membrane is an essential criterion for the manufacturing of real-time sensor devices. Silicon nitride (Si3N4), which is a traditional thin semiconductor film and sensing membrane, has been used for the development of biosensors.7 However, the surface oxidation of silicon nitride transferred into the silicon oxynitride limits its application to use as a sensing surface for fabrication of a solid-state EIS pH sensor.
In order to develop a pH sensor for commercial applications, various metal oxides (PdO, ZnO, WO3, and SnO2) have been studied for detection of pH.8–11 These materials contribute to enhancing the sensitive performance of sensor; however, they fail to address their problems like poor stability, lack of repeatability, high background to noise ratio, and lack of thermal stability, which ultimately restrict their applications.12 Many other high dielectric constant (high-κ) materials, including Al2O3/SiO2, CeO2, Ta2O5, TbTixOy,13–16 have also been proposed and investigated for the fabrication of high stable biosensor application due to their good sensing performances and high thermal stabilities. In the last few years, among these high-κ dielectrics, yttrium oxide (Y2O3) has gained considerable attention due to its good thermal stability (2410°C),17 high-κ value (∼15–18),18 large bandgap (5.5 eV),19 large conduction band offset (∼2.3 eV),20 chemically inert, and low lattice mismatch with silicon.21 These numerous advantages of Y2O3 thin film make an appropriate candidate for development of various applications, such as anti-reflective and protective coating,22 solid oxide fuel cell,23 electroluminescent devices,24 and catalyst.25 Additionally, the oxygen vacancies are the most common defects during the deposition of Y2O3 on a Si substrate, which affects overall the device response and its characteristic.26 In order to suppress the oxygen vacancy defects, the incorporation of TiO2 or Ti was used to obtain a stoichiometric Y2O3 thin film. Lee et al. proposed the insertion of Ti in the Y2O3 dielectrics to achieve a lower leakage current, a higher capacitance value, a lower electron trapping rate, and enhanced the structural integrity of Y2O3 film on a Si substrate.27 A considerable amount of research has been dedicated to the deposition of Y2O3 film by different technologies, such as atomic layer deposition (ALD),28 radio-frequency sputtering,29 metal-organic chemical-vapor-deposition (MOCVD),30 and e-gun evaporation.31 As compared to these methods, the sol-gel method has a number of attractive features which include control composition of binary-tertiary oxide, atomic level of mixing, economically profitable, low processing temperature, and low synthesis time. To date, our previous studies have investigated the uniform fabrication and super-Nernstian response of various kinds of sol-gel derived rare-earth oxide materials, such as PrTixOy,32 Ce2Zr1-xTixOy,33 Ce0.9 Sr0.1(Zr0.53Ti0.47)O4,34 based EIS sensor for the detection of pH. The present research explores, for the first time, the effect of annealing temperature on the structural and sensing characteristics of high-κ YTixOy sensing membrane for development of a solid-state EIS pH sensor. We used X-ray diffraction (XRD) to evaluate the crystal structure, atomic force microscopy (AFM) to investigate the film morphology, X-ray photoelectron spectroscopy (XPS) to determine the film composition. Furthermore, we coordinate the observed structural properties of YTixOy sensing membranes with their pH sensing characteristics.
Experimental
Preparation of YTixOy sol-gel solution
All the material and precursor solutions used in this research were of analytical grade. In order to prepare the YTixOy based sol-gel, yttrium oxide (Y2O3 granule, Admat Midas Inc., purity 99.99%) was dissolved in 2 N HCl solution to achieve a final concentration of 0.1 mol/L. Further, clear and transparent Y2O3 sol-gel were placed in water bath at 80°C and stirred constantly for 3 h. Subsequently, titanium precursor solution (titanium isopropoxide, [(CH3)2CHO]4Ti, Sigma Aldrich, purity 97%) was added in the Y2O3 sol-gel by dropwise under vigorous stirring. After the addition of titanium isopropoxide, the reaction is allowed to proceed up to 30 min. To obtain a stable homogeneous solution, lactic acid (0.5 ml) was added into the solution acting as a sol stabilizer. However, glycerol (1 ml) also was added into the solution to ensure the good spin condition like film thickness and proper adhesion on the Si substrate. This sol-gel solution was kept at ambient temperature for 48 h for aging so as to acquire the optimal viscosity during the deposition of the film.
Fabrication of sol-gel based EIS sensor and material characterization method
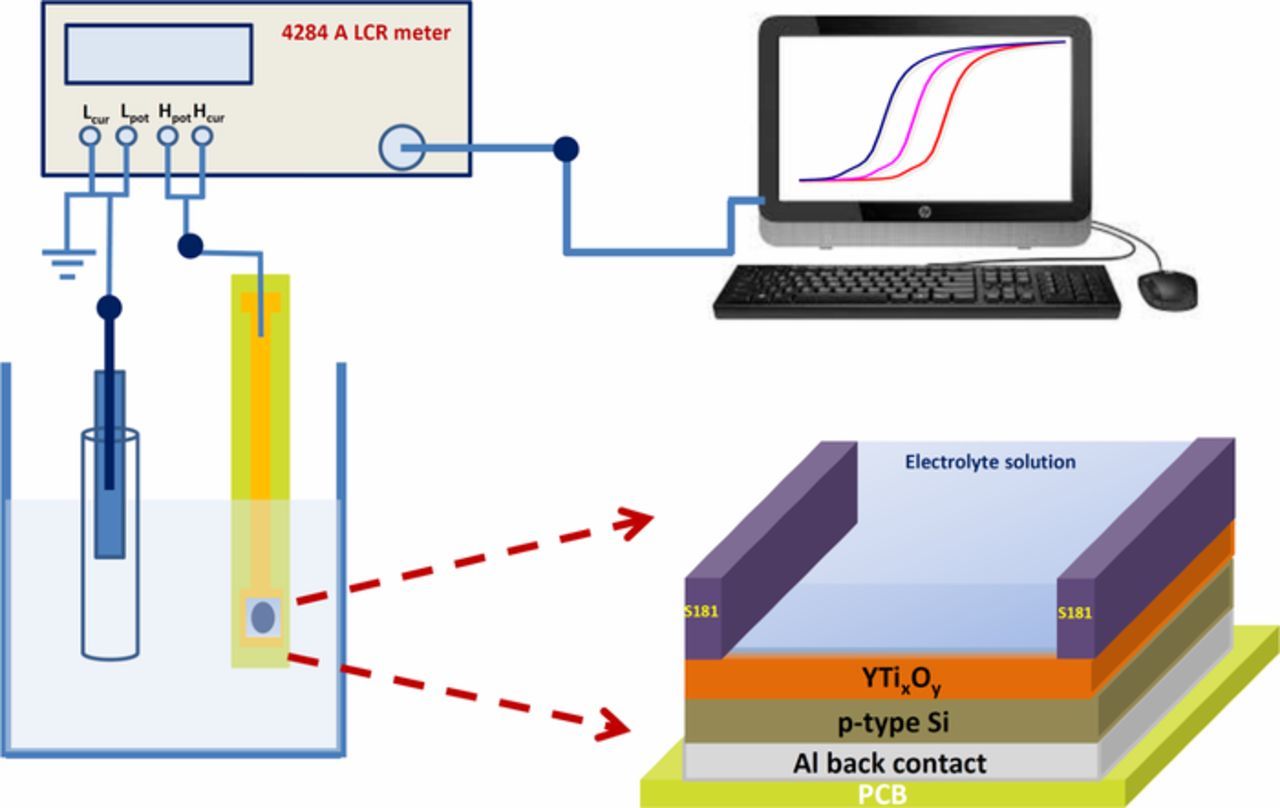
P-type (<100>) Si wafer with a resistivity of 1–10 Ω-cm was used for the fabrication of YTixOy sensing film based EIS sensor. Prior to the deposition of sensing film, the wafer was cleaned by a standard RCA cleaning procedure and further submerged into 1% HF solution to remove the native oxide. A ∼ 40 nm YTixOy film was deposited on the cleaned Si wafer by a spin coating technique with the spin rate of 3000 rpm for 30 s under suction. To enhance the adhesion between the YTixOy thin film and Si substrate as well as the evaporation of organic solvent from YTixOy films, the deposited film was dried at 300°C for 5 min. Further, the YTixOy films were then underwent for annealing in a furnace with various annealing temperatures from 700 to 900°C in the presence of O2 atmosphere for 45 min. In order to establish ohmic contact, an aluminum film (∼100 nm) was deposited on the back side of silicon wafer by a thermal evaporation method. The sensing region of the YTixOy film was defined by using epoxy gel, which acts as an isolation layer. Finally, EIS structure was assembled on the copper line of a printed circuit board by the silver gel. To prevent the leakage of electrolyte solution, epoxy was used to distinguish the EIS structure and Cu line. Three devices on each annealing temperature were measured to evaluate sensing performance of the YTixOy EIS sensors. The schematic structure of YTixOy based EIS sensor is illustrated in Fig. 1.
Figure 1. Schematic illustration of an YTixOy EIS sensor and its measurement unit.
The electrical performance of YTixOy sensing membrane was evaluated by measuring capacitance-voltage (C-V) measurement of the prepared EIS devices in different buffer solutions with a Ag/AgCl reference electrode and HP4284 LCR meter (ac signal frequency: 500 Hz). All electrical experiments were performed in a Faraday cage to avoid the light and noise interference. The crystal structure and orientation of the YTixOy films were carried out by a Rigaku D/MAX2000 XRD with Cu Kα (λ = 1.54176 Å) line. The surface morphology and roughness of the YTixOy films were observed using an NT-MDT Solver P47 AFM with a silicon probe operated in tapping mode. The root-mean-square (Rrms) variation of the height values was calculated from 3 × 3 μm2 scan areas. The chemical bonding of the dielectric film was studied using a Physical Electronics Quantum 2000 XPS instrument equipped with a monochromatic Al Kα X-ray (1486.7 eV) source. The symmetric Gaussian–Lorentzian product functions were used to approximate the line shapes of the fitting components. Peak fitting was performed after Shirley background subtraction.
Results and Discussion
Structural properties of YTixOy sensing membrane
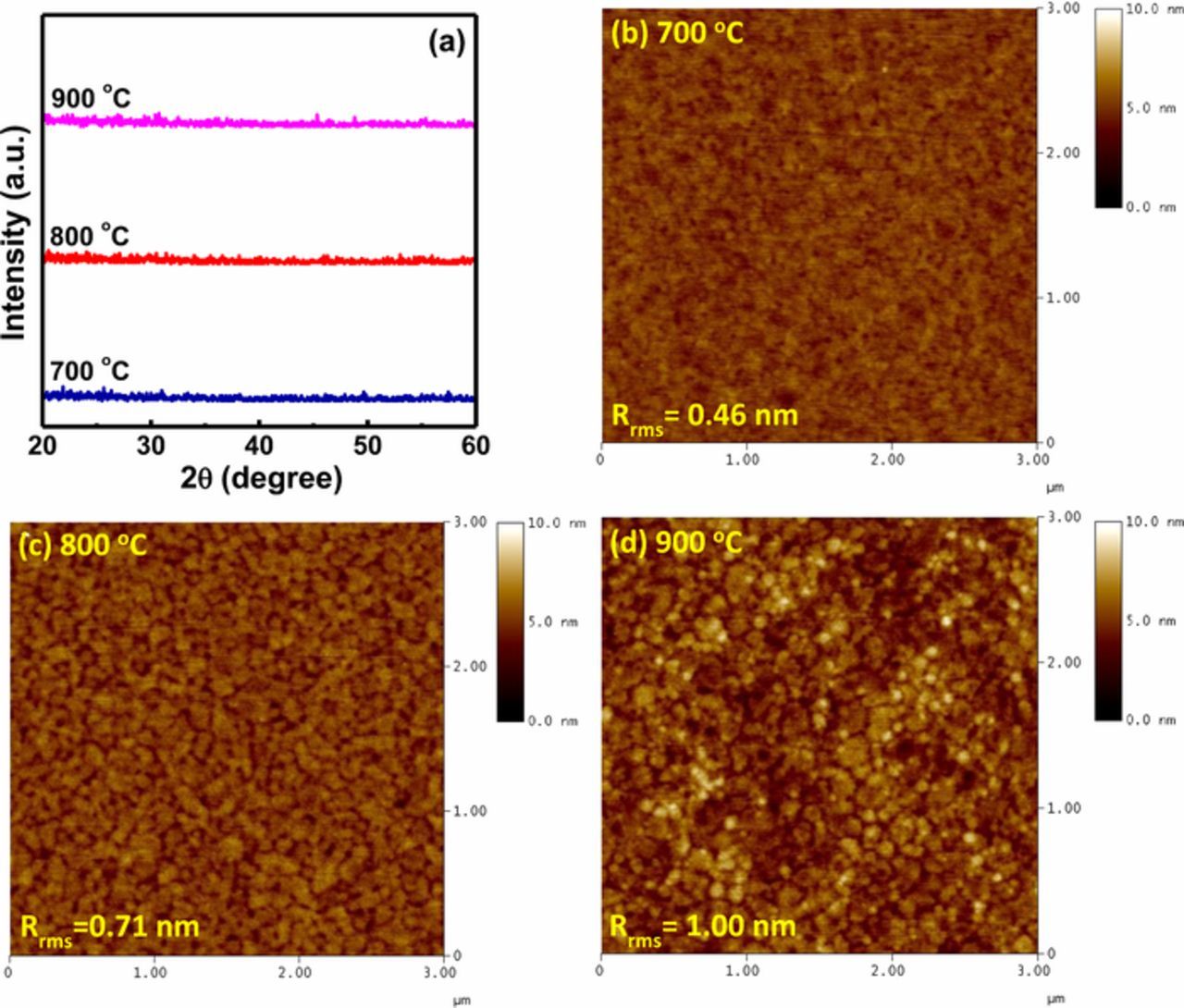
In order to understand the effect of annealing on the orientation and crystalline nature of YTixOy sensing films, XRD measurements were performed as shown in Fig. 2a. The sample performed at annealing temperatures of 700, 800 and 900°C, no apparent crystalline phase was observed and the pattern showed a large diffuse background, suggesting that all annealed YTixOy films exist in the amorphous structure.
Figure 2. (a) XRD pattern of YTixOy sensing membranes annealed at different temperatures. AFM surface images of YTixOy sensing membranes after annealing at (b) 700 (c) 800 and (d) 900°C
The surface morphology is an important characteristic of sensing membrane for assessment of its performance in the electrolyte solution. To obtain the surface roughness and grain size of sensing films, the annealed YTixOy sensing membranes were explored from AFM images in Figs. 2b–2d. The surface roughness values of 0.46, 0.71 and 1 nm were obtained for the YTixOy sensing membranes annealed at 700, 800 and 900°C, respectively. From the obtained morphology, we can clearly observed that the surface roughness increased with the degree of annealing temperature. This property ascribes that the native grains are coalesced and organized into larger grain due to the post-annealing treatment. This divergent nature of larger grain influences the surface morphology of the sensing membrane. Furthermore, the sample annealed at 900°C showed more rougher surface, indicating that yttrium and oxygen atoms diffuse from the film and relocate toward the interface where it forms yttrium silicate (YSixOy) due to the interaction between the Y2O3 and SiO2.35
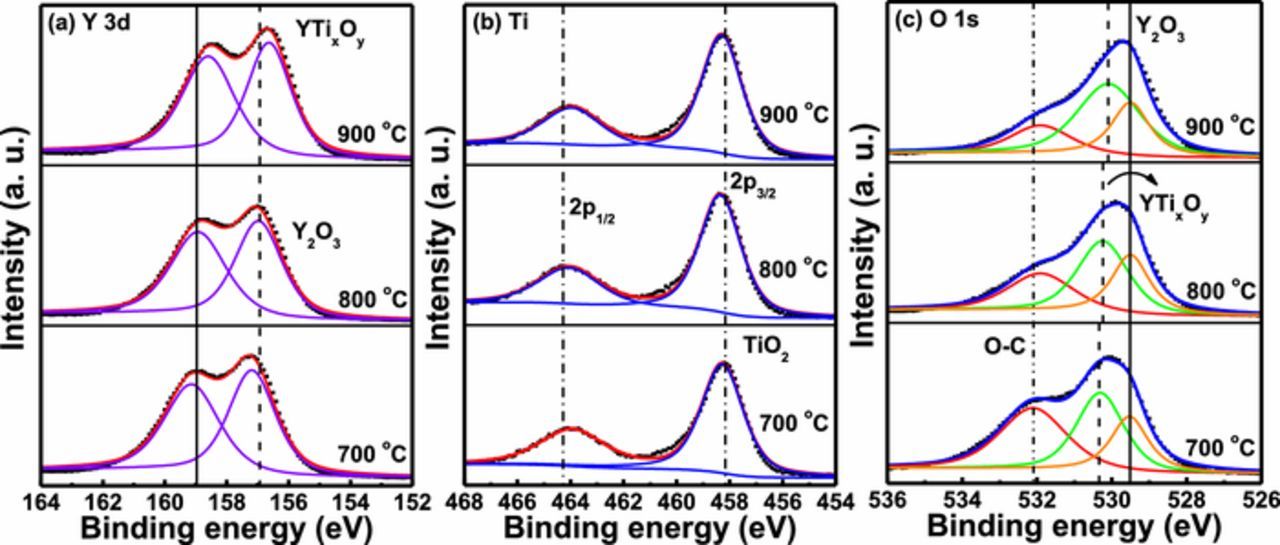
Impact of annealing temperature on the surface composition and chemical state of the YTixOy sensing membrane was further investigated by using XPS technique. Figs. 3a–3c show the Y 3d, Ti 2p and O 1s spectra of the YTixOy sensing films treated at different annealing temperatures, respectively. A signification variation in the peak position and intensity caused by a conventional furnace anneal is attributed to the surface modification in sensing membrane. As shown in Fig. 3a, the binding energy of Y 3d5/2 and 3d3/2 double peaks for the sample after annealing at 700°C located at 157.2 and 159.1 eV, suggesting weak (YTi)O2 structure formed on the surface of sample due to the subjection to air.36 The YTixOy sensing film annealed at 800°C, the binding energy of Y 3d5/2 and 3d3/2 double peaks shifted toward lower binding energies, appeared at 157 and 158.9 eV, respectively.27,28 Moreover, it was found that the increasing of annealing temperature at 900°C, Y 3d doublet peak shifted toward more lower binding energies by 0.3 eV. The position of Y 3d5/2 and 3d3/2 double peaks was obtained at 156.7 and 158.6 eV, respectively. It is found that the increase in annealing temperature have adverse effect on the surface. Thus, this finding suggests that the Y and O atom diffuse toward the interface and further intermixed with Si atoms, which facilitate the formation of an Y-silicate layer at high temperature annealing. The XPS spectra of Ti 2p3/2 and 2p1/2 double peaks for the YTixOy films annealed at different temperatures were presented in Fig. 3b. The peak position of Ti 2p3/2 and 2p1/2 at 458.3 and 464 eV, respectively, for sample annealed at 700°C is assigned for Ti4+ in the YTixOy film.37 It was also observed that the binding energy of Ti 2p3/2 peak increased from 458.3 to 458.4 eV with increasing in the annealing temperature from 700 to 800°C, respectively, arising from Y-O-Ti bond. This shift also evidenced the similar phenomenon as described above, the Y atom moved toward interface to make silicates, while the incorporation of Ti leads to the formation of Y-O-Ti bond which is more mechanically stable. The O 1s spectra for the YTixOy films as a function of annealing temperature are shown in Fig. 3c, with three peak curve fitting lines. In the three peak sets, one peak at 529.1 eV represented the O atom in Y2O3,38 another peak at 529.9 eV corresponding to a weak Y-O-Ti bond was observed for the 700°C-annealed sample, and the other peak at 531.7 eV can be regarded carbon contamination in sample. In contrast, the increase in annealing temperature at 800°C the binding energy of O 1s peak shifted toward a lower binding energy by 0.1 eV (529.8 eV), indicating that Ti replaces the oxygen and forms stoichiometric Y-O-Ti bond. Additionally, further increment in annealing temperature at 900°C, the binding energy decreased (529.7 eV), there is the diffusion of Y and O atom toward the interface to induce the formation of an interfacial layer. This observation suggests that the YTixOy sensing membrane annealed at 800°C suppresses the formation of an interfacial yttrium silicate, thereby leading to a stronger stoichiometric YTixOy structure.
Figure 3. XPS characterizations of (a) Y 3d (b) Ti 2p and (c) O 1s for YTixOy sensing membranes after annealing at various three temperatures (700, 800 and 900°C).
Sensing characteristic of YTixOy EIS sensor
To understand the sensing mechanism of an ISFET or EIS sensor, it is important to consider the presence of electrolyte between an insulator film and reference electrode. Moreover, Equations 1 describes the flatband voltage (VFB) of an EIS sensor which is defined as:39
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/166/4/B187/revision1/d0001.gif)
where ϕSi is the work function of silicon, Cox and Qox are the oxide capacitance and accumulated charge in the oxide, respectively, q is an elementary charge, and Qss is the surface charges and interface states in the oxide. The presence of electrolyte solution generates three different kinds of potential termed as Eref is the constant reference electrode potential, χsol is the surface dipole potential of the solution, and ψo is the surface potential associated with the function of solution pH, representing the flatband voltage of an EIS sensor. The site binding theory model has been proposed to understand the change in the surface charge of an oxide insulator in a liquid electrolyte. The difference in the size and charge of ion elicits the formation of the electrical double layer at the interface of an oxide membrane and electrolyte solution. As per this model, amphoteric insulating surface exhibited the hydroxyl group which can be protonated (Si-OH2+) or deprotonated (Si-O−) relying on the proton concentration in the buffer solution. Both protonation and deprotonation are responsible for the change in the surface potential of an oxide insulator. For this phenomenon, H+ and OH− ions are called potentially determining ions. This model was widely accepted for an EIS structure, however, the protonation and deprotonation are described as follow:
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/166/4/B187/revision1/d0002.gif)
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/166/4/B187/revision1/d0003.gif)
where [H+s] ion is the concentration of proton, which exists at the surface of an oxide insulator, and [SiOH2+], [SiO−] and [SiOH] are the binding site for proton concentration near the interface.
According to Boltzmann distribution, [α] is a concentration of ion species which exist at location i in the electrical double layer. [α]i compared to bulk concentration [α]b is described as follow:
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/166/4/B187/revision1/d0004.gif)
where kB is Boltzmann constant and T is the absolute temperature. In chemical reactions, the surface potential of the electrolyte is related to the charge density accumulated on insulator's surface. The net surface charge density (σ0) is given by
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/166/4/B187/revision1/d0005.gif)
While the total density of binding sites is
![Equation ([6])](https://content.cld.iop.org/journals/1945-7111/166/4/B187/revision1/d0006.gif)
The obtained voltage (ψ0) dependent on the sensing membrane material and the pH value of an electrolyte solution is explained by using SiO2 and Al2O3 two surface models proposed by bousse et al., hence combining Equations 2–6, the calculated surface potential is:40
![Equation ([7])](https://content.cld.iop.org/journals/1945-7111/166/4/B187/revision1/d0007.gif)
where pHpzc is the pH value with zero charge and β is the dimensionless pH sensitivity parameter which determines the chemical sensitivity of sensing membrane and depends upon the density of surface binding site as showing in the Equation 8:
![Equation ([8])](https://content.cld.iop.org/journals/1945-7111/166/4/B187/revision1/d0008.gif)
where CDL is the double layer capacitance (based on Gouy-Chapman-stern model which the value is 20 μF/cm2). According to the theoratical model if β >> 1, the maximum sensitivity of an EIS sensor is 59.2 mV per unit of pH (25°C), which termed as Nernstian response. Apparently, from Equation 8, it is theorized that the pH sensitive performance and linearity of the sensing membrane are proportional to the number of the surface site per unit area. However, the density of surface site is firmly associated with a surface roughness of the film. Change in the H+ ion concentration results in the VFB shift in the capacitance-voltage (C-V) curve.
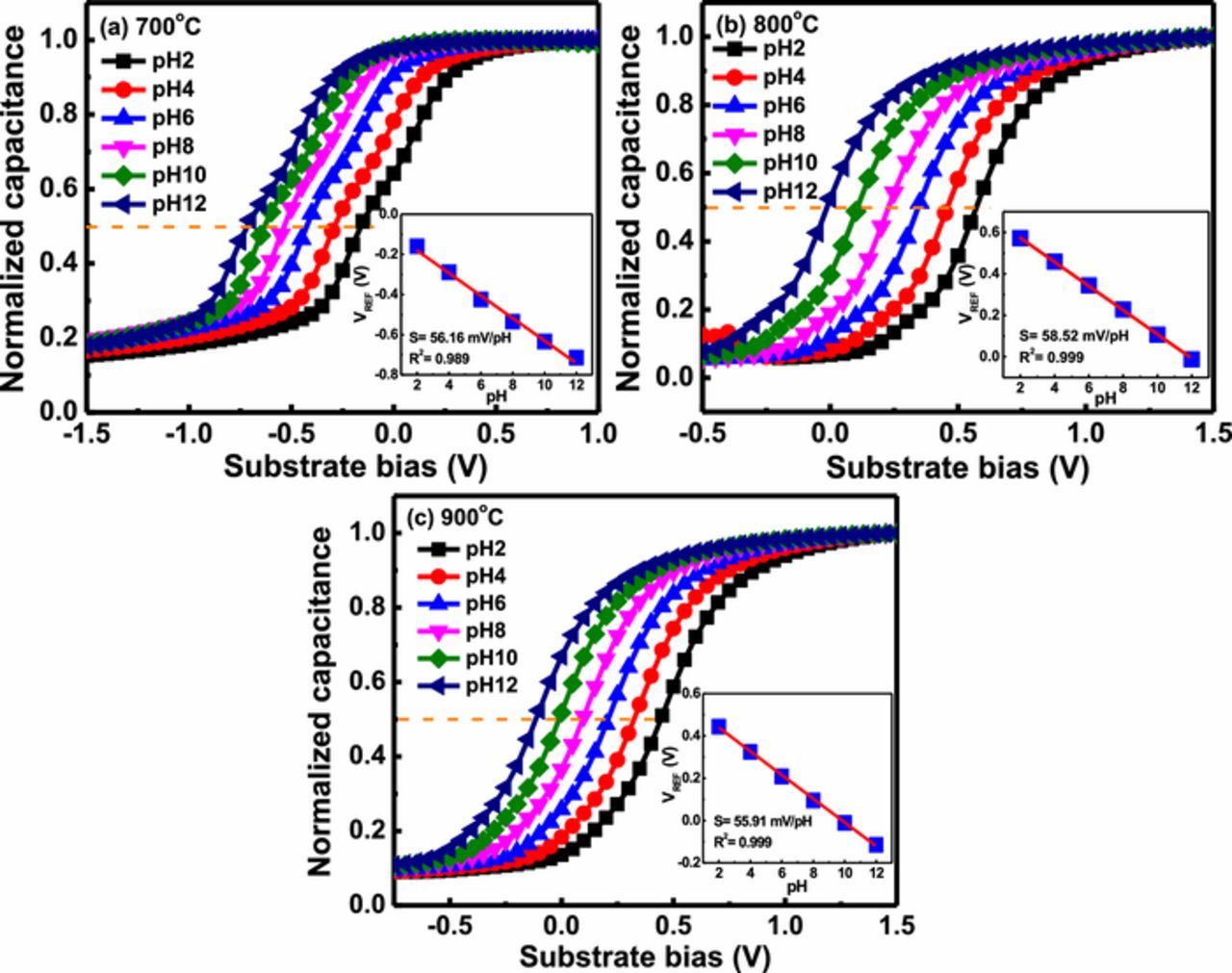
In order to investigate the effect of different annealing temperatures (700, 800 and 900°C) on the sensing characteristics of YTixOy sensing membranes, we quantified C-V curves at pH ranges from pH 2 to 12, as shown in Figs. 4a–4c. From the obtained C-V curve, the VFB shifted toward the negative side as the pH of buffer solution increased due to the lowering of surface potential as described in site binding model previously. To measure the pH sensitivity of YTixOy based EIS sensor, the significant change in the voltage region at 50% of maximum capacitance at each buffer solution compared to detect the corresponding hydrogen ions concentration. However, to calculate the linearity of YTixOy EIS devices which fabricate with different annealing temperatures, reference voltage (VREF) as a function of pH was plotted in the inset of Figs. 4a–4c. The pH sensitivities of YTixOy sensing membranes after annealing at 700, 800 and 900°C were determined to be 56.16 ± 0.41, 58.52 ± 0.17 and 55.91 ± 0.11 mV per unit of pH, respectively. Among these tested devices, the YTixOy EIS device annealed at 800°C exhibited the highest sensitivity. The possible mechanism of the high sensitivity could be associated with the surface roughness of YTixOy sensing membrane as observed in previous AFM analysis (see in Fig. 2c). With increment in the surface roughness of YTixOy sensing membrane, it effectively increases the total surface area at insulator/electrolyte interface, which results in sensing membrane accommodating more charge (H+ or OH−) on its surface, hence pH sensitivity was improved at 800°C devices as compared to other samples. Moreover, 900°C-annealed device exhibted a lower sensitivity compared to 800°C in spite of having higher surface roughness, because at 900°C a solid state reaction takes place in which yttrium and silicon atom intermixed with each other, hence leading to the formation of a thicker silicate layer with a lower dielectric constant.41
Figure 4. C- V curves for pH sensing response of YTixOy sensing membranes annealed at (a) 700 (b) 800 and (c) 900°C. Inset: Reference voltage as a function of pH for YTixOy sensing membranes annealed at (a) 700 (b) 800 and (c) 900°C.
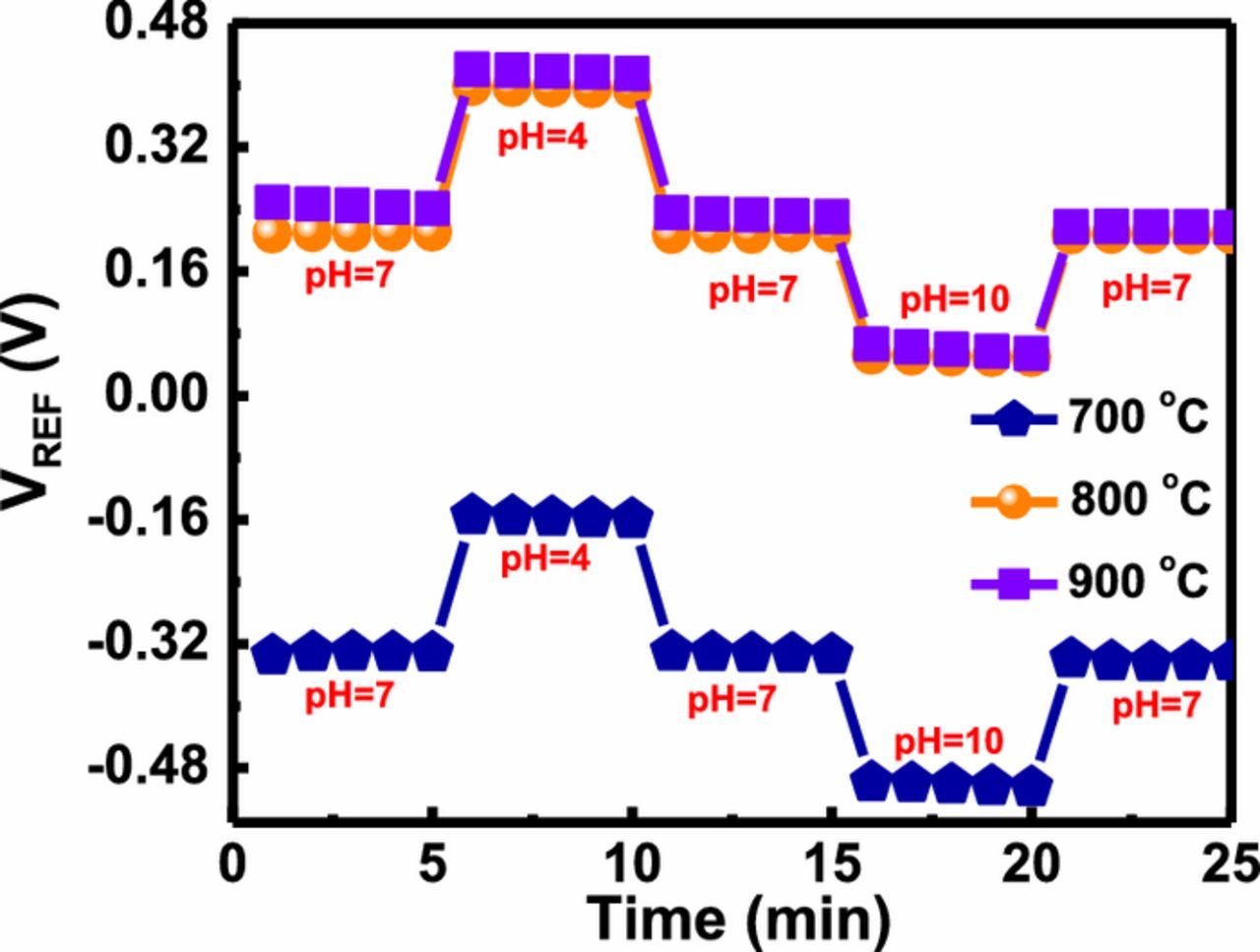
For the development of solid-state EIS sensor devices, reliability and stability are the most important factor have to be considered. In order to evaluate the stability of an EIS sensor, hysteresis phenomena have been performed. The hysteresis effect is also known as a non-ideal effect, which is mainly due to the slow interaction between the interior surface sites and ions in electrolyte solution. These interior surface sites are presented beneath the sensing surface, which mostly are defects. The hysteresis voltage described as the voltage difference between the initial and terminal voltage average, when YTixOy sensing membrane was submerged in an alternative cycle of pH loop 7→ 4→ 7→10→7 over a period of 25 min in each buffer solution. The hysteresis voltages of YTixOy based EIS sensors annealed at 700, 800 and 900°C are 27.6, 2.6 and 10.4 mV, respectively. Thus, the sample annealed at 800°C was shown a small hysteresis deviation (2.6 mV) in Fig. 5. Since the oxygen vacancies and various defects in sensing membrane are responsible for binding of ion on surface site of the membrane, which ultimately detains the reference voltage. Annealing at 800°C in the presence of oxygen ambient condition possibly saturates the oxygen vacancies and several defects, while increasing in annealing temperature 900°C evokes the diffusion yttrium and oxygen ion at their respective atomic positions which generate dangling bond and interfacial trap, this self-diffusion process leads to the formation of non-stoichiometric YTixOy, thus is responsible for a higher hysteresis voltage compared with 800°C-annealed film.
Figure 5. Hysteresis characteristics of YTixOy sensing membranes prepared at different annealing temperatures (700, 800 and 900°C) in pH loop of 7→ 4→ 7→10→7.
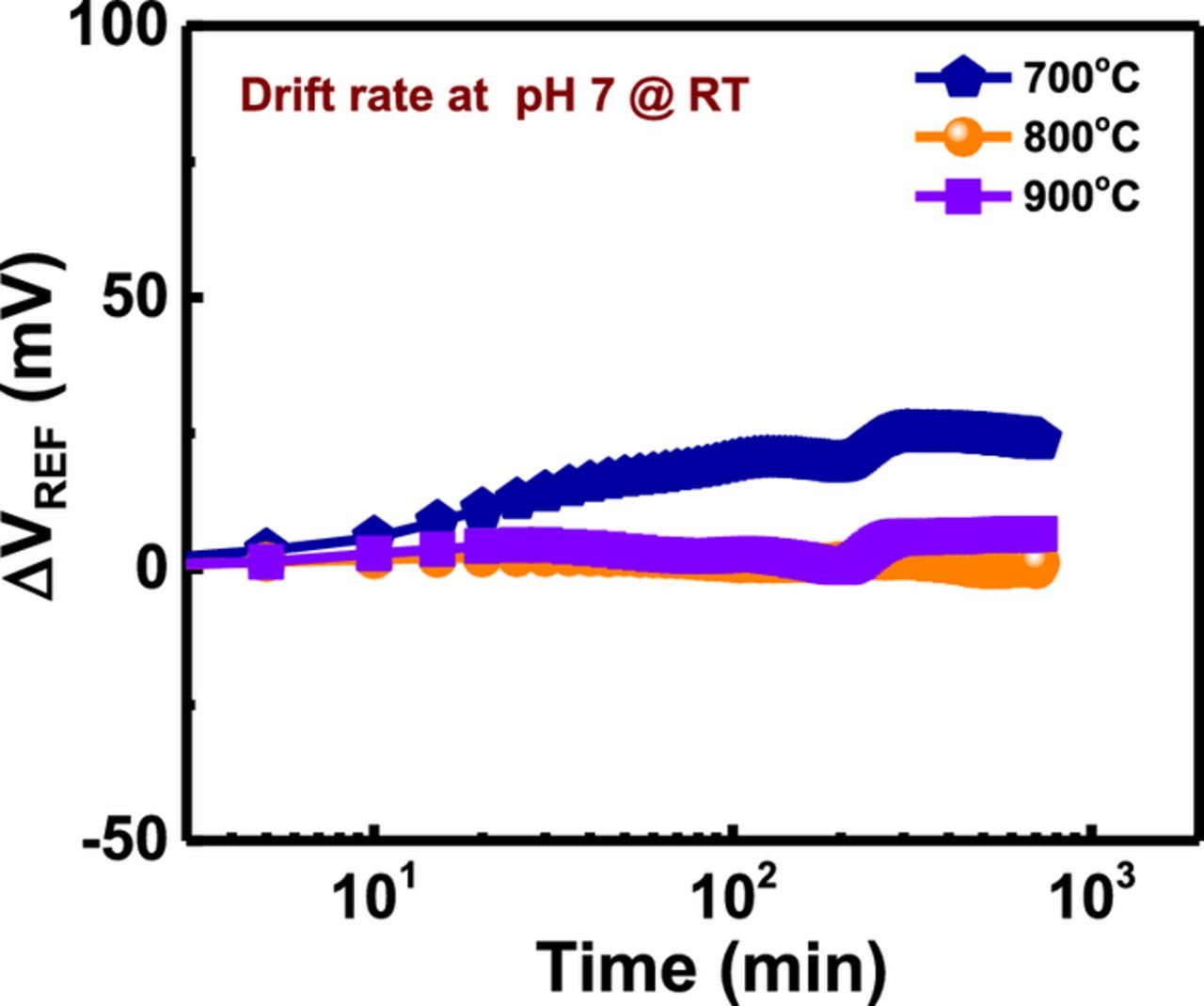
For the long-term measurement process, drift rate is a very essential parameter to examine the stability of EIS sensor devices. When EIS sensor is used for the detection of pH or biological molecule in the electrolyte solution, a comparatively slow chemical alteration occurred on the sensing membrane surface due to its vulnerability in the electrolyte solution. These chemical alterations occurred in the sensing membrane surface are responsible for the modification in the density of surface binding site after a period of time. This complete phenomenon has a deleterious impact on the overall capacitance of sensing membrane, which resembles the relatively slow, monotonic and temporal change in measurement circuit and the reference voltage. Impact of annealing temperature (700, 800 and 900°C) on the drift characteristics of YTixOy based EIS sensors depicts in Fig. 6. The samples were submerged in pH 7 solution for 12 h. Change in the reference voltage (ΔVREF) is described as:
![Equation ([9])](https://content.cld.iop.org/journals/1945-7111/166/4/B187/revision1/d0009.gif)
The drift rates of YTixOy EIS devices after annealing at 700, 800 and 900°C were found to be 1.98, 0.10 and 0.54 mV/h, respectively. This obtained result indicates that the sample annealed at 800°C had a smaller drift as compared to other annealed samples. The smaller drift rate at 800°C is possibly attributed to the presence of a lower crystal defect, oxygen vacancy, and dangling bond, accountable for trapping of H+ and OH− ion on the surface in the electrolyte solution, thus this optimal temperature prevents the clustering of ions. However, a larger drift occurred at high annealing temperature (700°C) rate may be due to more C-O bonds in the film to enhance the formation of crystal defect and buried surface sites, which are also known as trap centers or trap sites. This trapping causes the reference voltage shift over a period of time, which may worsen the drift response. In this investigation, we found that the sol-gel derived YTixOy EIS sensor displayed better pH sensitivity performances (sensitivity, hysteresis voltage and drift rate) as compared to other ISFET or EIS sensors composing HfO2 and ZrO2 (55 mV per unit of pH, NA and NA),42 Ta2O5 (56.19 mV per unit of pH, NA and NA),15 WO3 (50.2 mV per unit of pH),43 IGZO (53.3 mV per unit of pH, NA and NA),44 SiO2 (52.4 mV per unit of pH, 2.3 mV and 3 mV/h),45 Al2O3/SiNw Nano-ribbon (53.3 mV per unit of pH, NA and NA),46 IrOx (51.7 mV per unit of pH, 16.5 mV and 0.3 mV/h),47 and SnO2 (57.36 mV per unit of pH, 2.9 mV and 6.73 mV/h)48 sensing membranes.
Figure 6. Drift rates of YTixOy sensing membranes annealed at different temperatures (700, 800 and 900°C) and measured in solution at pH 7.
Conclusions
In this work, we investigated the effect of annealing temperature (700, 800 and 900°C) on the YTixOy sensing membranes prepared by the simple Pechini sol-gel method. Surface orientation, morphology, and composition were characterized by XRD, AFM, and XPS respectively. The pH sensitivity performance of YTixOy sensing membrane had been annealed at 800°C exhibited a higher pH sensitivity (58.52 mV per unit of pH), a lower hysteresis voltage (2.6 mV) and a smaller drift rate (0.10 mV/h) in comparison with other annealed samples. The obtained pH sensitivity is comparable to theoretical Nernstian response (∼59.2 mV per unit of pH). This condition can enhance the performance of YTixOy sensing membrane due to the lowering in lattice defect, oxygen vacancy, and surface roughness. These results indicate that the optimization of annealing temperature for the YTixOy EIS sensor can be applied as a stable pH sensor for the detection of various biological molecules in the medical diagnostic industry.
Acknowledgments
The authors thanks Ministry of Science and Technology of Taiwan for Financial supporting this research under contract of MOST-106-2221-E-182-063.
ORCID
Tung-Ming Pan 0000-0002-1013-2571