Abstract
This paper presents a fully inkjet-printed electrochemical sensor on paper which consists of carbon nanotube-printed working, reference, and counter electrodes. The proposed technique aims at low-cost and disposable paper-based electrochemical sensors. First, a carbon nanotube (CNT) ink was inkjet-printed directly on paper, forming a conductive network. Additionally, a hydrophobic barrier was patterned on paper to limit the absorption of liquid to the designed area. The inkjet printing method allows for rapid patterning of electrodes on paper, resulting in a simple and effective electrochemical sensor. The sheet resistance of the CNT-printed paper was as low as 1 kΩ/◻ after 33 prints. A potential step voltammetry method was applied to determine the concentration of the analytes, iron ion (Fe2+) and dopamine (DA), with linear ranges of 10 μM-200 μM and 10 μM-100 μM, respectively. The reported approach for a fully inkjet-printed electrochemical sensor is easy and cheap, and it has a potential for simple and rapid paper-based point-of-care diagnostics.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
Paper-based chemical and biological sensors have become attractive in recent years due to their facile fabrication, simplicity of use, and the ability of paper to easily absorb and retain liquid.1,2 Recent developments incorporate hydrophobic barriers,3,4 microfluidic channels5 and stacking layers for sensing purposes.6 Among other characteristics, paper is a low cost material, readily available, and is also flexible.
In order to develop a paper-based sensor, various materials must be patterned on paper for sensing purposes.7 Therefore a quick and easy method to pattern such materials is the subject of great interest. In connection with printed electronics technology, various printing methods exist for patterning conductive materials on thin substrates.8 Among them, inkjet printing has much interest due to many advantages such as automated printing process, mass producibility, and uniform deposition of materials.9 Here, we are interested in utilizing the inkjet printing technology for the development of a paper-based electrochemical sensor.
Printing methods have been applied for chemical sensors in a number of ways; however, since further steps were required in order to build a complete device, it is desirable to develop a simpler printing process. For example, inkjet printing was used to print dissolvable materials in order to pattern hydrophobic areas,10 where it was necessary to dip coat the paper and clean the dissolved material afterwards. In another instance, inkjet printing of metal nanoparticles was performed with an additional sintering step.11 Thus, a simpler one step process to develop a paper-based sensing device is necessary which should be easy and cheap to fabricate.
Although paper based analytical devices have been greatly advanced, a simple method of fabrication coupled with reliable detection method is still needed. Our approach targets the fabrication issue by integrating all the fabrication steps into a single method of inkjet printing. This can be achieved by using carbon nanotubes as a printing material: they are able to be inkjet-printed as ink solution while being able to form a percolated network on paper. Thus, carbon nanotubes can be directly used as electrodes on a paper substrate.
In addition to patterning carbon nanotube ink, we showed electrodes composed uniquely of carbon nanotubes. Traditional approaches for electrochemical techniques include modifying electrodes with carbon nanotubes along with other materials for improving the selectivity and/or the sensitivity.12–16 Those methods usually focus on the modification of the working electrode,17 while still using Ag/AgCl as the reference electrode. Our objective is to simplify fabrication by using the same material (CNT) as the working, reference and counter electrodes. By simplifying the fabrication steps, cost is reduced and this technique becomes more accessible and on-demand.
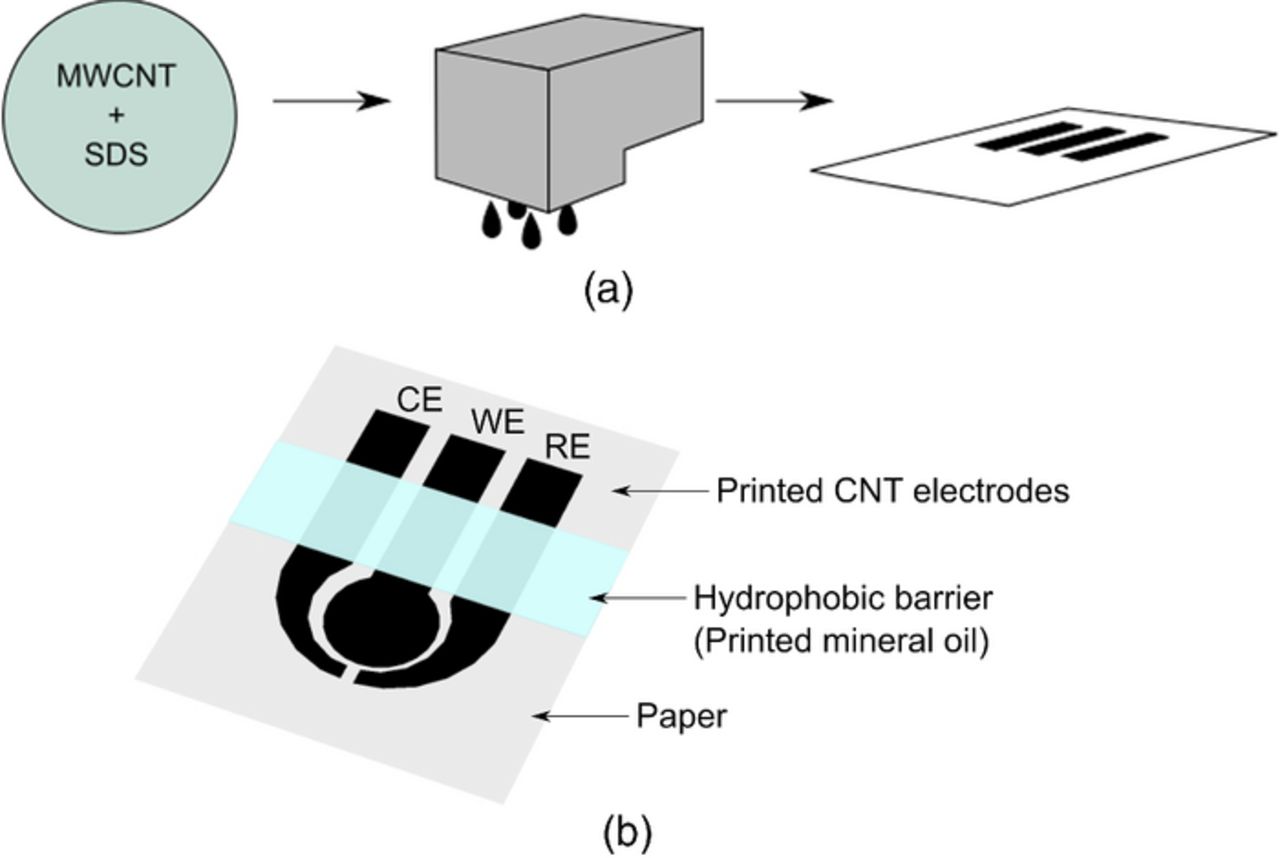
In our previous work, we have showed the printing characteristics of carbon nanotubes (SWCNT) in a flexible substrate (PET).9 Particularly, carbon nanotubes formed a conductive network that allowed redox reactions to occur and electrical current to be sensed by instrumentation. In this work, we further developed the concept by inkjet printing carbon nanotubes on paper and verifying that the generated network was conductive and was able to perform as electrodes for chemical sensing. As can be seen in Figure 1, the fabrication process consists of formulating the carbon nanotube ink and using an office inkjet printer to deposit and pattern the CNTs on paper. Once ready, the sensor is dipped into solution and voltammetry is employed to determine target analyte concentration (Figure 2).
Figure 1. Illustration of the fabrication process: (a) mixing of carbon nanotube ink by using optimal MWCNT-SDS ratio in deionized water, followed by inkjet printing the carbon nanotube ink, and (b) the sensor composed of counter, working, and reference electrodes, with a hydrophobic barrier on top.
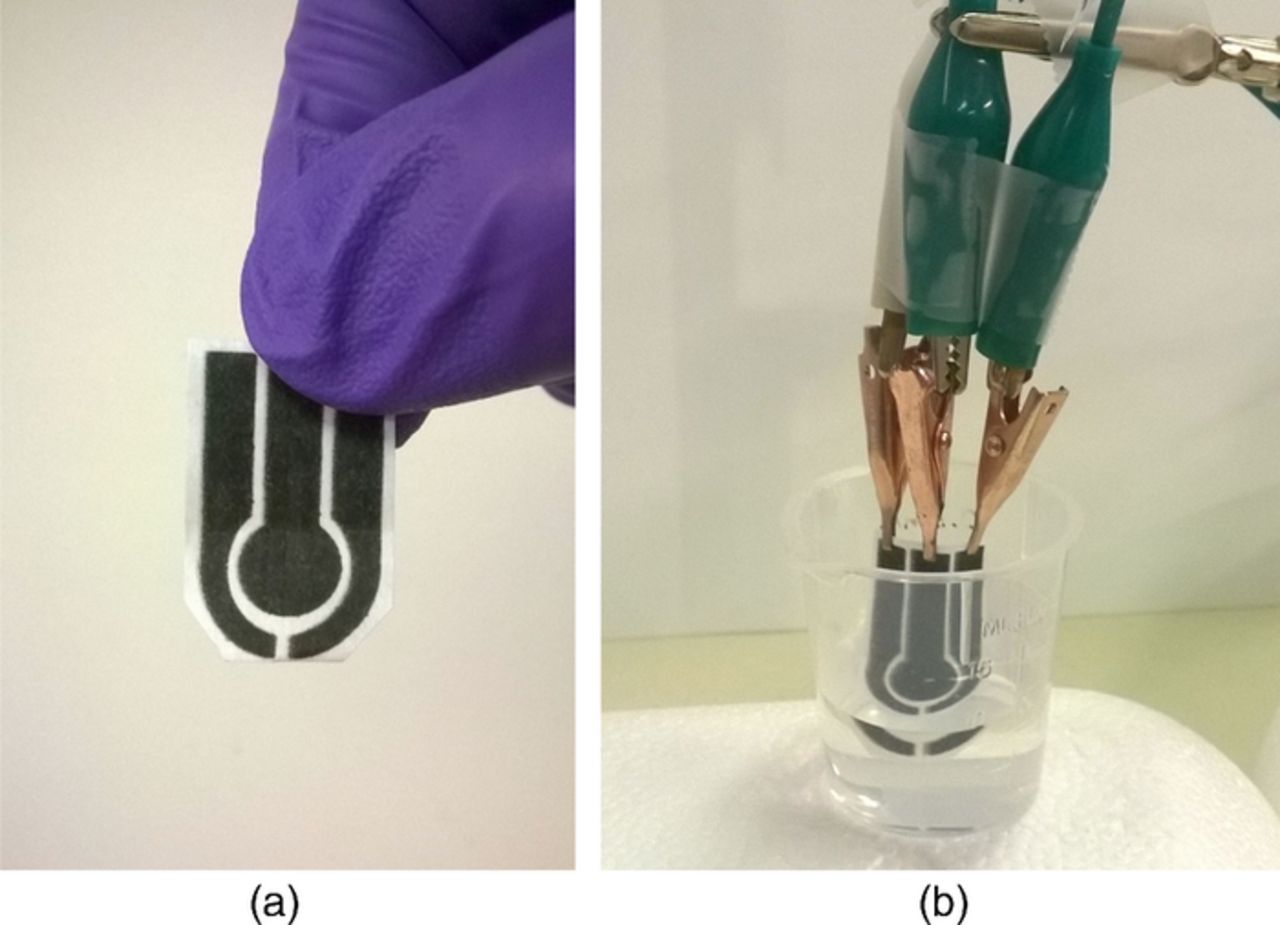
Figure 2. Pictures of the electrochemical sensor: (a) device ready for use and (b) sensor dipped into solution of iron sulfate.
This work presents the fabrication steps of the electrochemical sensor, including inkjet-printing of a carbon nanotube ink on paper. Also, its applicability is demonstrated for a compound that plays an important role in humans, which is the neurotransmitter dopamine. The sensors fabricated using this technique can easily be translated for mass producible scale and are able to perform in low limit of detection by using the electrochemical method. Therefore, the present technology aims to develop a simple and economical approach for electrochemical sensing of different species.
Experimental
Carbon nanotube ink preparation and printing
Carbon nanotube ink was formulated using the ink recipe developed in the previous work of our group,9 with the following concentrations: 1 wt% (10 mg/ml) of multi-walled carbon nanotubes (MWCNT) (CheapTubes Inc.), 0.7 wt% (7 mg/ml) of sodium n-dodecyl sulfate (SDS) (Alfa Aesar) and DI Water. The ingredients were weighed in a 20 ml vial and sonicated for 30 minutes in order for the SDS to disperse the carbon nanotubes. The SDS also lowers the surface tension of the water, thus optimizing the dispensing of ink by the cartridge's nozzle. The dispersion was transferred into centrifuge tubes and centrifuged for 5 minutes at 12,000 rpm in order to separate the large agglomerates to the bottom of the tube. The supernatant was recovered using a syringe and subsequently injected into the printer cartridge. Figure 1a shows the steps for making the ink, inserting into the cartridge, and inkjet-printing of the carbon nanotubes.
For inkjet-printing, an office HP Deskjet D4260 was used. First, an empty cartridge was opened and thoroughly cleaned. Then, CNT ink was injected directly to fully soak the sponge within the cartridge. Once the cartridge was inserted into the printer, simple CAD software was used to draw sensor patterns and print them on paper.
For the sheet resistance measurement, rectangular CNT lines of 2 mm × 20 mm (10 squares) were printed from 5 times up to 33 times, with an increment of 2. Between each print, 3 minutes were allowed to dry the ink.
Paper-based electrochemical sensor
Device design
In order for the chemical reaction rate to be maximized, a large working electrode area is desired. For this reason, the working electrode area was maximized, being 62.2 mm2, as illustrated in Figure 1b. Each electrochemical sensor used in this work had the same working electrode area. For the electrochemical sensors, all CNT patterns were printed 23 times.
Test solution preparation
Test solutions were prepared as follows: for sulfuric acid, 98.2 μl of 95% sulfuric acid was diluted in 350 ml of deionized water to create a 5 mM sulfuric acid concentration. For solutions containing iron, 55.6 mg of iron(II) sulfate heptahydrate (Fluka) was dissolved in 100 ml of 5 mM sulfuric acid to create 10 mM iron sulfate concentration. A series of dilutions of 10 mM iron sulfate was carried out to create the various iron concentrations. A pH 7 solution was made by an appropriate mixture of potassium phosphate monobasic (KH2PO4) powder and sodium phosphate dibasic anhydrous (Na2HPO4) powder. The dopamine solutions were prepared by dissolving dopamine hydrochloride powder in pH 7 buffer solutions.
Electrochemical measurement
Potential step voltammetry was carried out using a homemade potentiostat circuit containing a microcontroller coupled with a data acquisition system. According to the standard electrode potential for each solution, the corresponding voltage was applied stepwise from 0 V to the electrode potential. All experiments were carried out at room temperature.
Hydrophobic barrier patterning
After printing carbon nanotube electrodes, mineral spirit (used without modification) was inkjet-printed on top of the CNT electrodes to provide a barrier for absorption of liquid. With the use of hydrophobic barrier, the reaction area was maintained constant throughout the experiment time which allowed for consistent results to be obtained.
Results and Discussion
Device characterization and sheet resistance
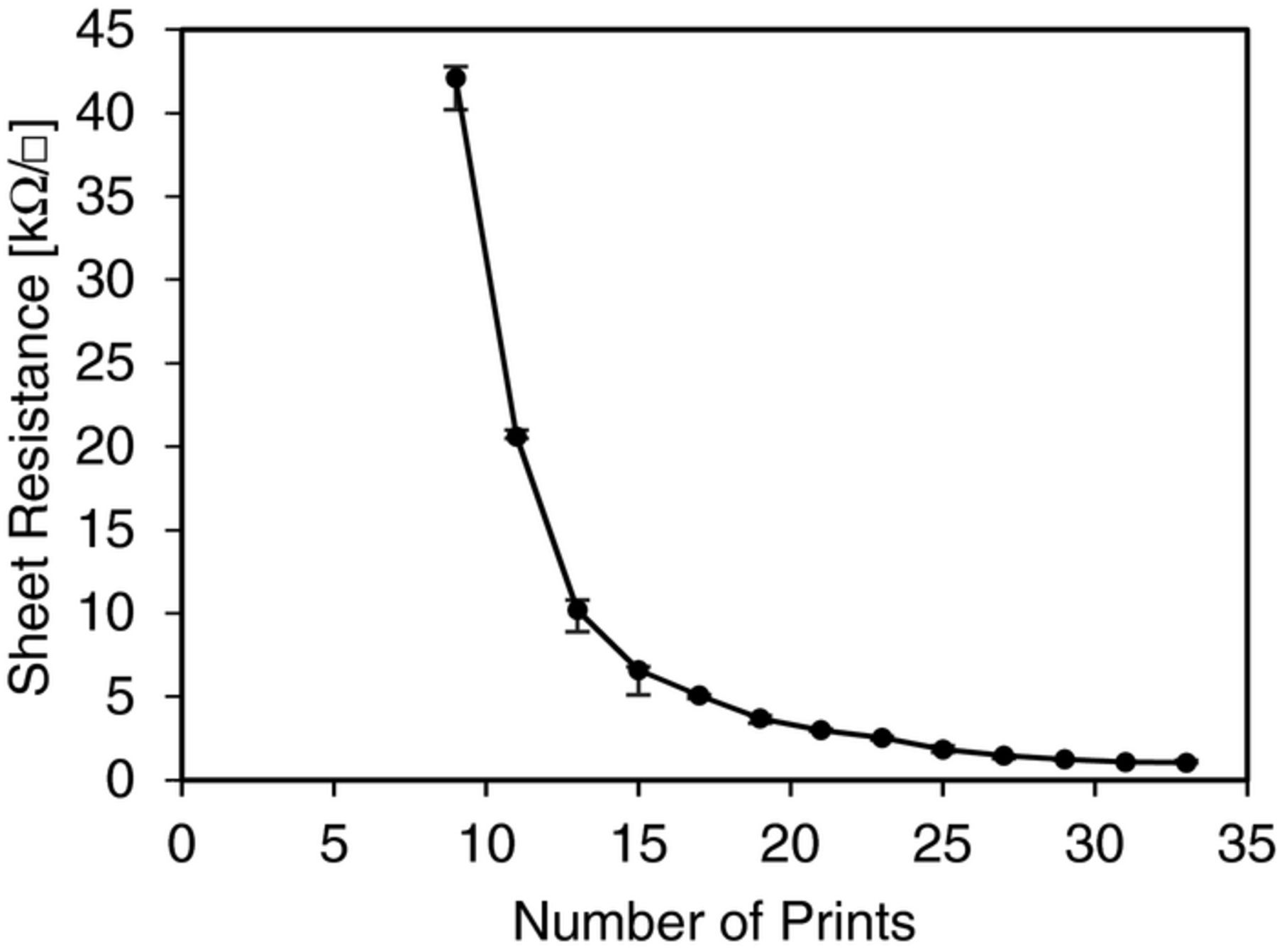
In order to work as an electrode, the carbon nanotube network is required to be conductive. The sheet resistance of the CNT network depends on how many sequential prints are performed: it decreases as the number of prints increases and becomes saturated, as shown in Figure 3. The lowest achieved sheet resistance was 1 kΩ/◻ for 33 prints. Considering the printing time and the decrease in sheet resistance, we found that a reasonably low resistance was achieved with 20∼25 prints for a given time. In addition, measured variations in sheet resistance were less than 5% after 20 prints for 10 different printed samples.
Figure 3. Carbon nanotube (MWCNT) sheet resistance according to the number of prints on paper. The lower limit was 1 kΩ/◻ for 33 prints. The CNT network starts to be conductive at around 9 prints.
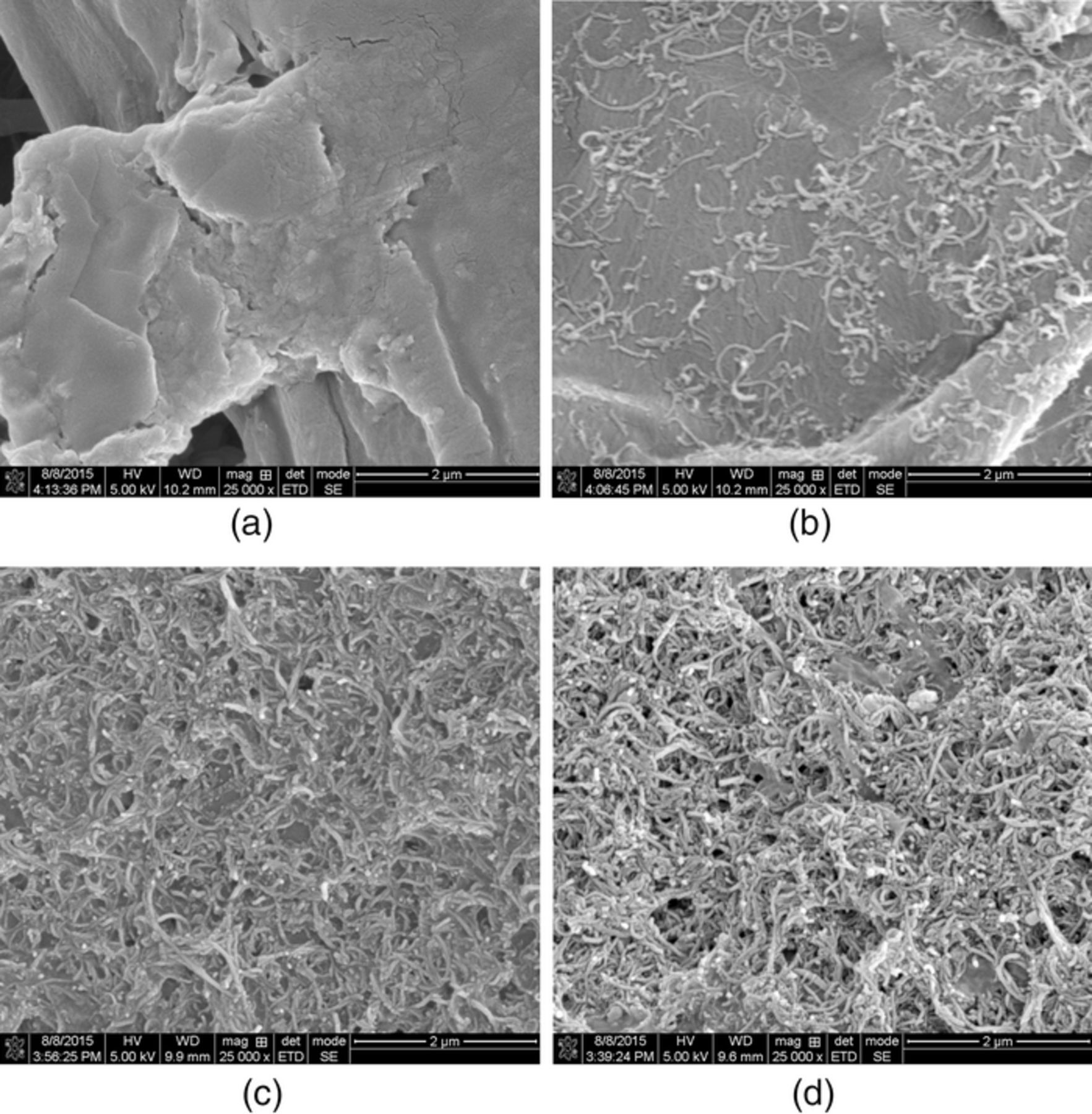
Figure 4 shows scanning electron microscopy images of printed carbon nanotubes on paper. It clearly shows that the carbon nanotubes start forming a network with 10 prints and become denser with a higher number prints.
Figure 4. SEM pictures of MWCNT deposited on paper: (a) pristine paper; (b) after 1 print; (c) after 10 prints, and (d) after 20 prints.
The electrodes were designed considering two important factors: the working electrode area and the resistance of the electrodes. First, the redox reaction of interest happens at the surface of the working electrode. Thus, its area was maximized in order to generate the maximum amount of electric current. This approach will improve the limit of detection of the sensor. Secondly, due to resistance of electrodes, the reference and counter electrodes are designed to wrap around the working electrode so as to maintain similar potential throughout the circumference of the working electrode. Therefore, the redox potential is maintained over the entire region around the electrodes.
As a measure of the resolution of the fabrication technique, the minimum conductive line width and the minimum spacing between lines were also measured. With our current inkjet printer used, we were able to achieve 0.7 mm for minimum line width and 0.7 mm for minimum spacing between lines. Printing conditions and parameters are still under optimization.
Characterization of the hydrophobic barrier
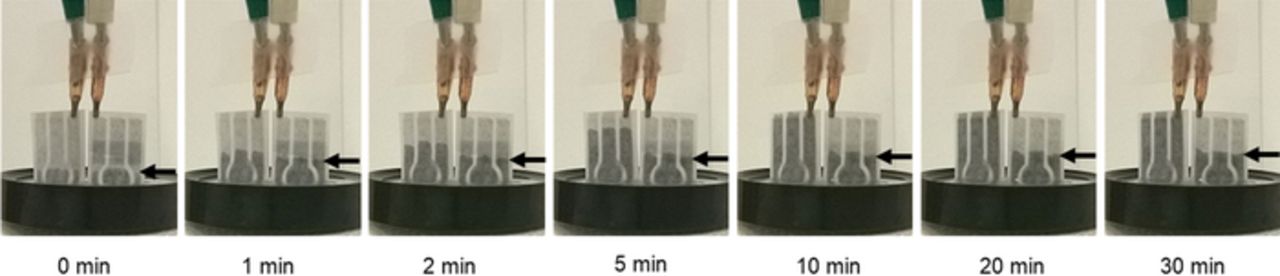
The hydrophobic barrier was inkjet-printed and characterized in terms of the spreading length over paper and time that it held the absorption of liquid. The designed printed area was 28 mm × 7 mm. The oil spread was between 0.2 mm and 0.3 mm. Additionally, the time that the hydrophobic barrier held the liquid was more than 30 minutes (time-lapse pictures are shown in Figure 5), which indicates that a potential step voltammetry measurement can be performed without adverse effects of water absorption.
Figure 5. Hydrophobic barrier test: liquid in a container is being absorbed by paper. On the left sensor there is no hydrophobic barrier and on the right sensor it is present. The hydrophobic barrier held liquid for more than 30 min.
Consistency of measurement across devices
After confirming that the carbon nanotube network was conductive, an electrochemical sensor was fabricated and characterized. In order to provide reliable determination of analyte concentration, the printed electrochemical sensors should give a consistent current response to a given concentration. The variation among the devices was tested with a set of potential step voltammery experiments and the settling current values were measured. The settling current determines the bulk concentration of the analyte (FeSO4) in solution. For each sensor, the concentration tested was 0.05 mM. Although there were variations in the settling currents among five different sensors, the concentration was consistently determined with a standard deviation of 0.027 μA for an average settling current of 4.38 μA.
Electrochemical characterization
To demonstrate the capability of electrochemical sensing, the potential step voltammetry method was applied to the sensor to determine the iron concentration. For this experiment, 5 mM sulfuric acid was used as supporting electrolyte and the potential was stepped from 0 V to 0.641 V (vs. CNT reference electrode). Upon the increase in potential between the working and reference electrodes, a sudden peak in current is observed. This peak occurs because all of the electroactive species present at the surface of the electrode is instantly oxidized. For the case of iron, the redox reaction at the working electrode is:
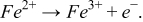
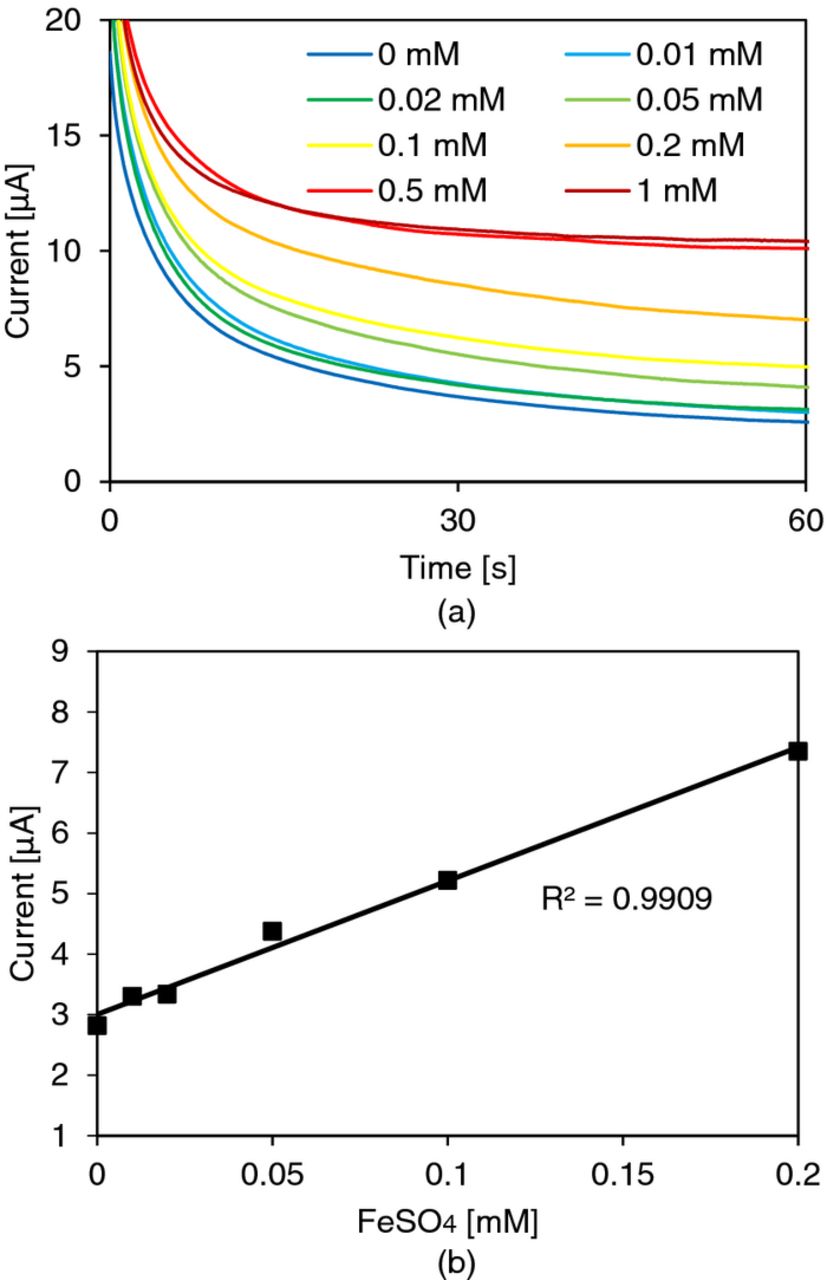
Once the reactions start to occur, Fe2+ is depleted at the surface of the electrode and it is replenished by the diffusion of the iron species from the bulk. Thus, current observed at the electrodes will be mass diffusion-limited according to the Cottrell equation.18 From the current information, it is possible to determine the concentration of target analyte in the bulk of the solution. We measured the concentration of Fe2+ and found a linear range from 10 μM to 0.2 mM as shown in the graphs in Figure 6.
Figure 6. Iron sulfate (FeSO4) measurement results: (a) potential step voltammetry of FeSO4 diluted in 5 mM sulfuric acid (H2SO4) and (b) calibration curve. The limit of detection is 10 μM and the area of the working electrode in contact with solution was approximately 62.2 mm2. The step potential was 0.641 V.
Application: dopamine detection
The developed inkjet-printed electrochemical sensors were also used for the determination of dopamine concentration. Dopamine is oxidized to dopamine o-quinone by a two-electron oxidation step. In this oxidation step, the electrons are donated to the CNT electrode and sensed as electric current,
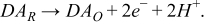
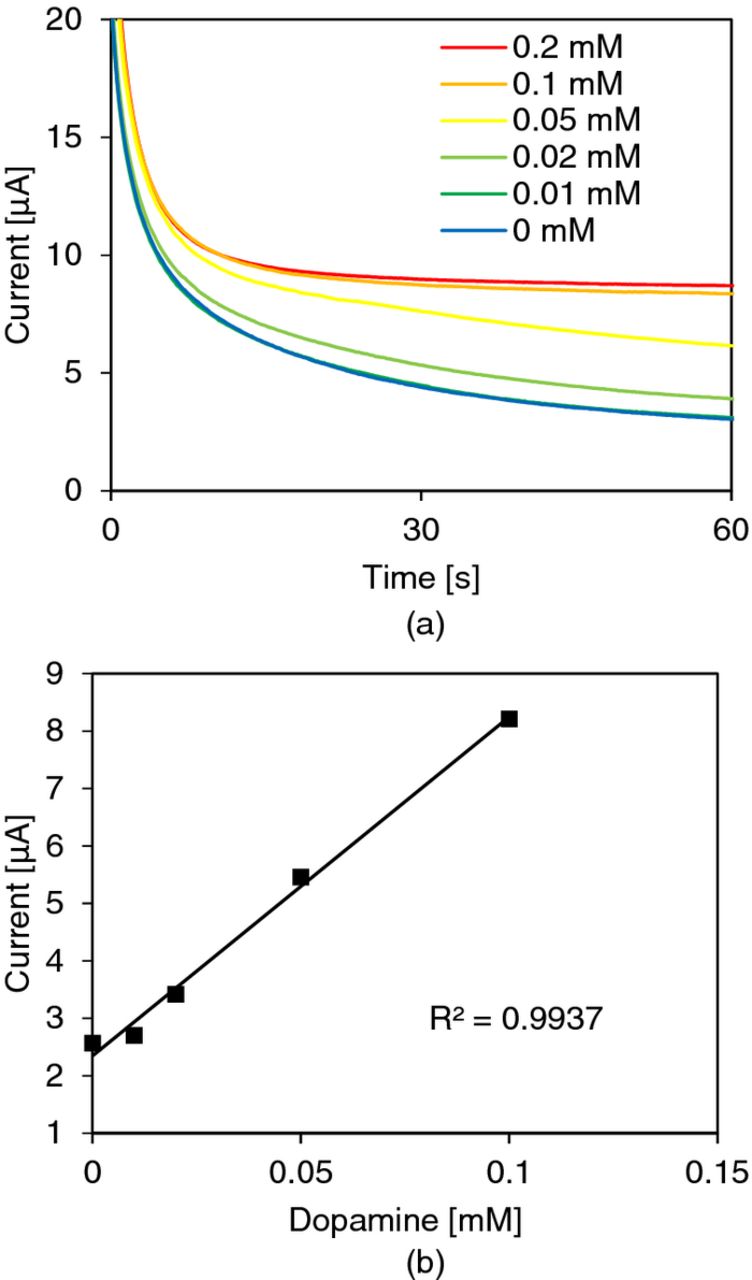
Although redox potential for dopamine is reported to be 0.752 V (vs. SHE),19 the optimal potential was found to be 0.69 V (vs. CNT electrode) in our case. We believe that the discrepancy between the redox potential is caused by the difference in reference electrodes and the ohmic voltage drop due to the resistance of the CNT working electrode. Figure 7a shows measured potential step voltammograms of dopamine. The linear range was found to be from 10 μM to 0.1 mM. The calibration curve was taken at 60 s from the step voltage input and showed an R2 value of 0.9937 for data in the linear range (Figure 7b). The result indicates that our paper-based sensor is able to provide a reliable measurement in spite of the simplicity in fabrication. The limit of detection could be lowered if a more sophisticated voltammetry technique were used, such as differential pulsed voltammetry.20
Figure 7. Dopamine measurement: (a) potential step voltammetry of dopamine in pH 7 and (b) calibration curve. The limit of detection is 10 μM, and the area of the working electrode in contact with solution was approximately 62.2 mm2. The step potential was 0.69 V.
Conclusions
We have successfully demonstrated the first fully inkjet-printed carbon nanotube-based electrochemical sensor on paper. Printed carbon nanotubes were used as reference, working, and counter electrodes. The sheet resistance of carbon nanotube electrodes was achieved less than 5 kΩ/◻ with over 20 prints. The minimum sheet resistance was 1 kΩ/◻ with 33 prints. We have demonstrated the applicability of the sensor to measure iron (Fe2+) and dopamine (DA) in concentrations as low as 10 μM. In addition to being cheap and easy to fabricate, it provides a simple readout system suitable for portable applications. The easy fabrication of this type of sensor is due to inkjet-printing technology, which provides rapid and low-cost patterning of carbon nanotubes on paper. The key advantage of this approach is to provide extremely low cost fabrication of paper-based analytical devices in conjunction with the simplicity of electrochemical detection.
Acknowledgments
This work was supported in part by the Fund for Innovation in Engineering Research, Economic Development Assistantship from Louisiana State University, IEEE Charles LeGeyt Fortescue Graduate Scholarship, and the CAPES Science without Borders Scholarship from the Ministry of Education of Brazil. The authors thank Dr. Junseo Choi from the Department of Mechanical and Industrial Engineering for his assistance in taking SEM pictures.